
Glycogen storage disease -
This division can also be found in all sorts of general papers on medical biochemistry, genetics, and in pediatrics or neonatology. Another attempt to classify GSDs is the Shin [ 21 ] study, where the glycogenosis classification is precisely categorized according to the enzymatic substrate of glycogen metabolism and the subtypes associated with certain organs, giving a total of 12 GSDs Table I.
Various types of GSDs types of GSDs according to tissue-specific enzymatic deficiency. Özen [ 17 ] classifies GSDs on the basis of the latest knowledge in the field, their enzymatic deficits or involved tissues, mainly on the basis of available case studies.
Case studies and data collection during patient health monitoring prove an increase in the number of GSDs studies [ 18 , 22 , 23 ]. The knowledge and awareness of these complex metabolic pathologies are increasing. The author divided the aforementioned disorders into hepatic, mixed, and muscular GSDs Table II [ 17 ].
Classification of GSDs into hepatic and muscle types with separate classification of PhK deficiency type IX. The basic clinical symptoms common for every type of hepatic GSD are hypoglycemia and hepatomegaly [ 24 ]. A characteristic feature for muscle GSDs is progressive muscle pathology, including exercise-induced muscle weakness [ 25 ].
Metabolic acidosis with hypercholesterolemia and hyperlipidemia are found in blood biochemistry [ 26 ]. In both types of GSDs, there is also hypertransaminasemia elevated activity of liver enzymes — aminotransferases. Physical development delay, mostly in the form of short stature or motor latency, is a typical clinical symptom.
GSDs are a group of heterogeneous genetic diseases; therefore each type has its distinct, specific clinical presentation [ 27 ].
Laboratory parameters such as increased lactate level in GSD type I , elevated level of serum cholesterol and triglycerides and hypertransaminasemia are helpful in diagnosis establishment.
Symptoms in GSD I typically present earlier in the first few months of life with severe fasting hypoglycemia within 3—4 h after feeding. Blood β-hydroxybutyrate levels increase only modestly in GSD I, in contrast to marked hyperketonemia with fasting hypoglycemia characteristic of GSD 0, III, VI, and IX [ 29 ].
Other biochemical characteristics that help to distinguish between these disorders are elevated uric acid and lactate levels in GSD I, whereas these are typically normal in ketotic GSDs [ 30 ].
The final diagnosis is always established or confirmed with molecular tests. Liver biopsy, in order to assess enzymatic activity in hepatocytes, is no longer the gold standard — it has been replaced by genetic examination, which is non-invasive and gives a certain and final diagnosis [ 29 , 30 ].
The disadvantages of molecular testing are its still high costs, limited availability and long time of waiting for the results. Decomposition of glycogen molecules is directly related to energy production, and its regulation takes place by activation of the relevant substances and enzymes.
In a healthy body, glycogen metabolism is effective through the balance between glycogen synthase and phosphorylase activity Figure 2.
Both enzymes can be reversibly phosphorylated in more than one site by separate kinases [ 8 ]. Glycogenolysis branched glucose polymer phosphorolysis is carried out in three steps:. Glycogenolysis takes place in the liver, during hunger or in the muscles during intensive exercise, to compensate for a decrease in blood glucose levels or a lack of chemical energy carriers in cells — ATP [ 31 ].
In the early phase of glycogenolysis, the released glucose is already phosphorylated and can undergo further transformation.
In this state it cannot freely leave the cell, which makes it a very energetically beneficial reaction for the working muscle.
However, there are circumstances in which glycogen metabolism is inadequate. The enzyme occurs in many tissues, thus its subunits also have their specific isoforms. The activity of PhK has been studied in such organs as the liver, muscles, kidney, testes, heart and also in erythrocytes, leukocytes and nerve cells [ 32 , 33 ].
The PhK enzyme consists of 4 subunits: α, β, γ, and δ subunits, which are encoded by the following genes:. PHKB subunit β , PHKG1 and PHKG2 subunit γ expressed in the liver [ 34 , 35 ],.
CALM1, CALM2 and CALM3 subunit δ [ 32 ]. The cAMP-dependent protein kinase regulates the phosphorylation of the Ser residues in the α and β subunits. The γ subunit contains a catalytic site, and the calcium-binding subunit δ has an affinity for calmodulin [ 36 ].
Type IX is the only GSD that is X-chromosome linked recessively inherited: the α1 subunit is X-linked inherited, while the remaining subunit units are AR inherited [ 37 ].
Mutation in the PHKA2 gene is the most common cause of GSD IX [ 38 ]. This type of GSD is characterized by a relatively mild course. Tissues affected by the disorder, depending on the subtype, are liver, erythrocytes, kidneys and muscles [ 39 , 40 ].
The symptoms occur during infancy or early childhood, and include growth delay and liver enlargement [ 41 ]. Episodes of hypoglycemia or ketonuria without a reasonable cause are rare.
If present, they are associated with a prolonged state of starvation or increased physical activity. Lactic acid and urinary acid concentrations are usually normal, and metabolic acidosis with hypocalcemia is rare.
Hypertriglyceridemia and hypercholesterolemia, as well as various levels of hypertransaminases, are also observed: from slightly to several times elevated transaminases. It causes delays in motor development due to the possibility of spreading in the muscle tissue. These disorders normalize during adolescence [ 13 ].
Based on the additional involvement of the skeletal muscles and the heart, there are 2 subtypes of GSD III: hepatic-muscle type subtype a and a hepatic one subtype b. Like all other GSDs but type IX-α, it is inherited in an autosomal recessive manner; the mutation is in the AGL gene located on chromosome 1p21 [ 46 ].
The first signs of the disease appear in early childhood and include enlarged liver, delayed growth and physical development, whereas hypoglycemia neurohyglycemia is not that common.
As age progresses, the hepatomegaly reverses, and muscle weakness subtype IIIa progresses slowly. Other symptoms common to this type of GSD include muscle hypotension and cardiomyopathy.
Frequently, the symptoms regress during adolescence, except in rare cases when cirrhosis of the liver or myopathy occurs [ 47 ]. Type VI is a mild form of GSD. The enzyme block consists of decreased liver enzyme activity — phosphorylase involved in the glycogenolysis process. The disease is inherited in an autosomal recessive manner, with a mutation in the PYGL gene on chromosome 14qq22 [ 48 ].
Symptoms are the same as in type III. In this type of GSD the heart and skeletal muscles are never involved, and liver adenomas are very rare. The disease does not carry the risk of organ failure [ 49 ]. The enzyme defect consists of reduced glycogen brancher enzyme GBE activity, mutation in the GBE1 gene on chromosome 3p12, and autosomal recessive inheritance.
GBE deficiency results in the accumulation of abnormal forms of glycogen that resemble the amylopectin polyglucosan body structure. Hence, its other name is amylopectin or adult polyglucosan body disease APBD [ 50 ].
The symptoms are very heterogeneous and include both the liver and the neuromuscular system. Children are generally born healthy but, as early as in the first months of life, they develop hepatomegaly and hypotonia and their psycho-motor development is delayed.
The disease progresses rapidly. It leads to liver fibrosis and portal hypertension which manifests as ascites and eventually leads to death. However, several cases of non-available hepatic GSD IV have also been reported in the literature [ 51 ].
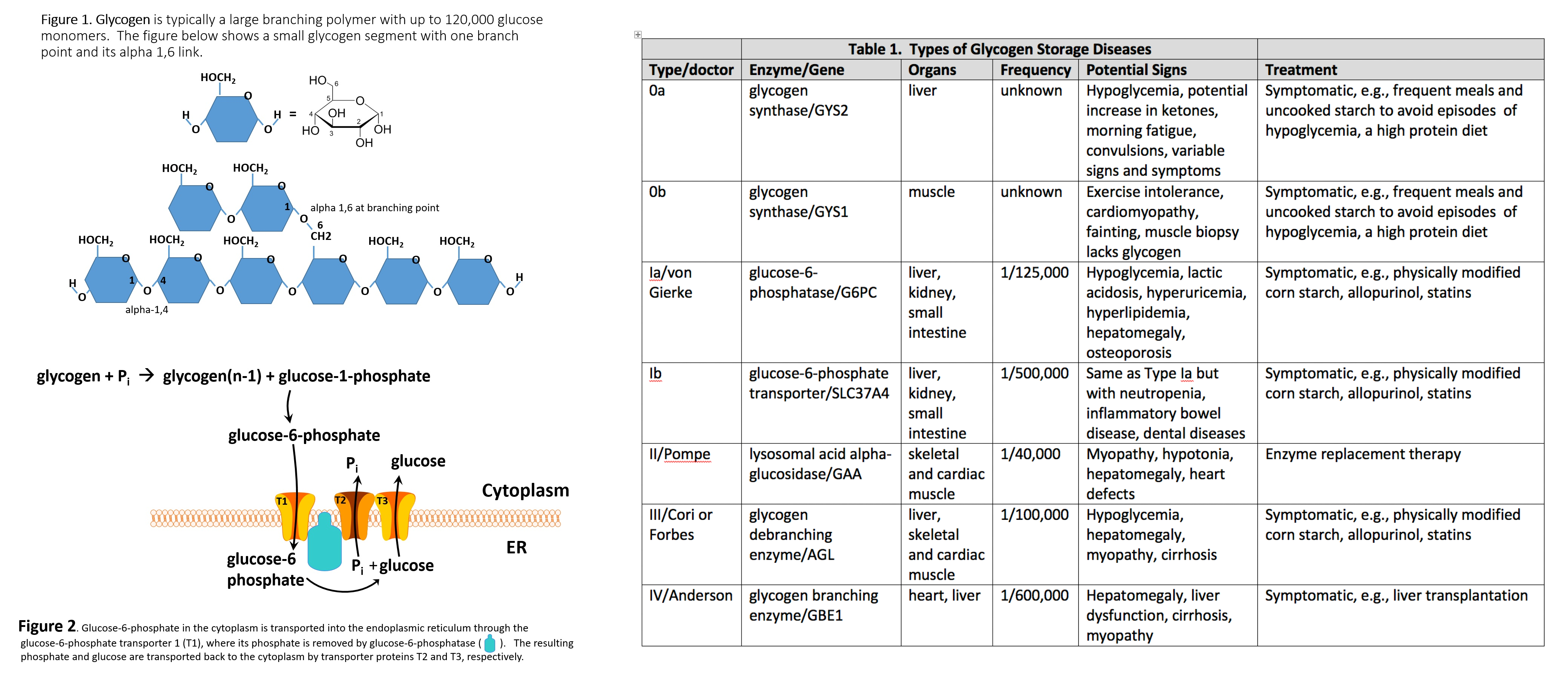
Unlike muscles, liver contains the glucosephosphatase membrane enzyme, which removes the phosphate residue to allow the glucose to enter the bloodstream and regulates its concentration [ 52 ].
Glucosephosphatase 1 G6PC enzyme catalyzes the hydrolysis of glucosephosphate to glucose, thus forming the last step of glycogenolysis and gluconeogenesis [ 53 — 56 ]. The G6PC enzyme is encoded by the G6pc gene, which is expressed in the liver, kidneys and pancreas, and its mutation is inherited in an AR pattern [ 52 , 57 ].
The function of the enzyme requires its translocation through the membrane of the endoplasmic reticulum. Another enzyme, G6PC translocase encoded by the SLC37A4 gene, is also involved in this process, which can also be mutated AR inheritance to impair the function of neutrophils, which is inscribed in the GSD type Ib.
After eating a meal, blood glucose rises. Hormone levels regulating these metabolic pathways are in inverse proportions, i. the concentration of glucagon decreases, and the concentration of insulin increases. There is no breakdown of glycogen then. The phosphorylation pathway of inactivated phosphorylase b to active a is off.
On the other hand, the pathway of phosphorylation of active synthase a to inactive b is activated. We are dealing with the postprandial state of glycogen synthesis stored as a backup material in tissues, primarily in the liver.
Before meals, when blood glucose levels fall, insulin levels drop, and another hormone, glucagon adrenalin-like , shows an upward trend. The glycogen degradation reaction to glucose is started. We obtain an active form of glycogen phosphorylase a and an inactive form of glycogen synthase b.
The basic treatment for GSDs is dietary management, which raises many controversies. Dietary recommendations differ slightly between the United States and Poland, but they are different in Europe, for example, comparing the UK and Poland. The aim of dietary treatment is to avoid hypoglycemia.
Therefore, it is necessary for patients to consume frequent meals during the day every 3—4 h with the addition of raw cornstarch RS and to shorten the night break by an additional portion of RS in the middle of the night [ 58 — 60 ].
Starch, like glycogen, is a polysaccharide — the process of releasing pure glucose into the bloodstream, in this case, is extended in time.
This results in constant access of substrates to the biochemical reactions of energy synthesis pathways, limiting its accumulation in tissues and thereby involving glycogen [ 61 , 62 ]. In the UK, overnight glucose infusions through a pump or probe are still used.
However, for patients with an unstable glucose level or ketosis, 2 g of unprocessed RS per kilogram of body weight is prepared to prevent morning hypoglycemia [ 63 ].
The situation is different for patients with type I GSD, who present with the most severe hypoglycemia and have most unstable glucose levels — both glycogenolysis and gluconeogenesis are impaired in this type and, additionally, ketogenesis is ineffective and there is a risk of neuroglycopenia.
Therefore, in GSD type I starch is a drug and not just a dietary supplement; it must be given regularly during the day every 3—4 h with a night break not exceeding 7—8 h [ 30 ]. Patients most often need to take an extra dose of starch at night while maintaining a constant supply of carbohydrates in a liquid form, especially during sleep, thus protecting patients with decompensated glycemia from dangerous complications of hypoglycemia.
When intolerance or reluctance to receive RS is observed, either a nasogastric tube or percutaneous endoscopic gastrostomy PEG is required — if the enteral supply is to be maintained for more than 6—8 weeks ESPEGHAN guidelines.
The disadvantage of starch is that it is hard to digest — with an infection associated with gastrointestinal irritation in children with glycogenosis there are often problems with starch supplementation.
It is not recommended for patients with type Ib due to their common inflammatory bowel disease and, therefore, additionally impaired absorption. The formula is used in patients older than 5 years old.
Currently, glycosade is to be studied in the United States for its application during the day in adult patients, in whom, due to the slower metabolism than children, the extended-release preparation might be effective not only at night.
According to current US dietary recommendations, the diet should be restricted to simple sugars less than 5—10 g in each meal in all types of GSDs, while it is most restrictive in type I, with RS supplementation in type I every 3—4 h daily plus an extra overnight dose or only 1 night dose in other types [ 64 ].
Because of the increased risk of micronutrient deficiencies vitamin D 3 , calcium-phosphate disturbances, and the risk of osteopenia , multivitamins and vitamin D 3 supplements are also recommended [ 30 , 64 ]. The basic principle of treatment is therefore to limit the simple sugars in a diet rich in complex carbohydrates.
However, the diet is always individually selected for the patient based on its glycemic status, current biochemical findings metabolic equilibrium and anthropometric parameters, and requires close collaboration between the physician, metabolic dietitian, the patient and his family.
Table III summarizes different dietary strategies in ketotic and non-ketotic type I GSDs. Summary of different dietary strategies in ketotic and non-ketotic type I GSDs. As mentioned above, a properly applied specific diet is a treatment in GSDs. It leads not only to stable normoglycemia, but also to decreased hepatomegaly reduction of glycogen storage , improvement of growth and biochemical metabolic control normalization of transaminases, triglycerides, in type I — reduction of lactate and uric acid in blood [ 13 ].
The problem of diet, as an important factor in treatment, may lead to eating disorders in children with GSDs. In the course of metabolic diseases, some eating disorders related strictly to the elimination diet can be observed. For example, people consuming frequent meals with high carbohydrate content that slows down the release of glucose into the blood may experience a lack of hunger.
Since a lot of attention is paid to eating, it may cause a lack of pleasure from eating a meal, not to mention the lack of taste of non-sweet foods. As far as children are concerned, following the heavy-starch diet is often associated with digestive problems.
Establishing the right diagnosis and setting a proper glycogenic diet will make the patients enjoy eating and discovering new flavors, changing the attitude to a meal as a necessity. In the treatment of eating disorders, therapies of these disorders adapted to the requirements of GSDs are helpful [ 28 ].
The GSDs, like all genetic diseases, is incurable. Currently, clinical trials on gene therapy for type I glycogenosis clinical phase of GSDI adult safety assessment are ongoing at the Connecticut facility in the US [ 66 ]. Currently, in the USA, the development of adeno-associated virus AAV vector-mediated gene therapy is being carried out for GSD type I based on the success of early-stage clinical trials of gene therapy in hemophilia [ 67 ].
At present, gene therapy for GSD type I is at the stage of a safety clinical trial on adult patients with this type of GSD, and is taking place in the Connecticut Hospital within the GSD program of Prof. The GSD is a congenital defect of carbohydrate metabolism characterized by hypoglycemia, hepatomegaly, and growth disorders short stature.
Basic therapeutic treatment consists in maintaining a proper diet with RS supplementation. Due to the fact that awareness and knowledge about rare diseases are still insufficient, it is important to popularize them among pediatricians, hepatologists and geneticists.
Knowledge of the biochemical basics and glucose metabolism in the human body facilitates proper treatment of GSD. Current issue Archive Manuscripts accepted About the Journal Editorial office Editorial board Abstracting and indexing Subscription Contact Ethical standards and procedures Most read articles Instructions for authors Article Processing Charge APC Regulations of paying article processing charge APC.
Manuscripts accepted. About the Journal Editorial office Editorial board Abstracting and indexing Subscription Contact Ethical standards and procedures Most read articles. Instructions for authors Article Processing Charge APC Regulations of paying article processing charge APC.
Current issue. Hepatic glycogen storage diseases: pathogenesis, clinical symptoms and therapeutic management. Edyta Szymańska 1.
Dominika A. Jóźwiak-Dzięcielewska 2. Joanna Gronek 2. Marta Niewczas 3. Wojciech Czarny 4. Dariusz Rokicki 1. Piotr Gronek 2. Laboratory of Genetics, Department of Gymnastics and Dance, University School of Physical Education, Poznan, Poland.
Department of Sport, Faculty of Physical Education, University of Rzeszow, Rzeszow, Poland. Department of Human Sciences, Faculty of Physical Education, University of Rzeszow, Rzeszow, Poland. Glycogen storage diseases GSDs are genetically determined metabolic diseases that cause disorders of glycogen metabolism in the body.
The first symptoms of the disease usually appear during the first months of life and are thus the domain of pediatricians.
Due to the fairly wide access of the authors to unpublished materials and research, as well as direct contact with the GSD patients, the article addresses the problem of actual diagnostic procedures for patients with the suspected diseases.
Knowledge and awareness of the problem among physicians seem insufficient, and research on the diagnosis and treatment of GSD is still ongoing, resulting in a heterogeneous GSD typology and a changing way of its diagnosis and treatment.
Figure 1 Cascade of glycogen breakdown. Glycogen storage diseases glycogenoses Pathophysiology and epidemiology Glycogen storage disease GSD is caused by a genetically determined metabolic block involving enzymes that regulate synthesis glycogenesis or glycogen breakdown glycogenolysis [ 13 ].
Inheritance Glycogen storage diseases, like most metabolic diseases, are inherited in an autosomal recessive AR way. Typology of GSDs It is still an unsettled matter.
Table I Various types of GSDs types of GSDs according to tissue-specific enzymatic deficiency. Table II Classification of GSDs into hepatic and muscle types with separate classification of PhK deficiency type IX.
Classic Infantile Juvenile Adult. Clinical symptoms The basic clinical symptoms common for every type of hepatic GSD are hypoglycemia and hepatomegaly [ 24 ]. Glycogen pathway Decomposition of glycogen molecules is directly related to energy production, and its regulation takes place by activation of the relevant substances and enzymes.
Figure 2 Enzymatic activity of phosphorylase and synthase. Release of glucosephosphate-kinase phosphorylase PhK. Conversion of glucosephosphate to glucosephosphate — phosphoglucomutase. Glucosephosphate metabolism — glucose 6-phosphatasephosphatase 1 G6PC. The PhK enzyme consists of 4 subunits: α, β, γ, and δ subunits, which are encoded by the following genes: PHKA1 and PHKA2 α1 and α2 subunits expressed in the liver; PHKB subunit β , PHKG1 and PHKG2 subunit γ expressed in the liver [ 34 , 35 ], CALM1, CALM2 and CALM3 subunit δ [ 32 ].
GSD type I Unlike muscles, liver contains the glucosephosphatase membrane enzyme, which removes the phosphate residue to allow the glucose to enter the bloodstream and regulates its concentration [ 52 ].
Dietary management The basic treatment for GSDs is dietary management, which raises many controversies.
Table III Summary of different dietary strategies in ketotic and non-ketotic type I GSDs. CS every 3—4 h during the day plus extra dose at night.
Usually only 1 dose of CS at night High-protein diet. Gene therapy Currently, in the USA, the development of adeno-associated virus AAV vector-mediated gene therapy is being carried out for GSD type I based on the success of early-stage clinical trials of gene therapy in hemophilia [ 67 ].
Conclusions The GSD is a congenital defect of carbohydrate metabolism characterized by hypoglycemia, hepatomegaly, and growth disorders short stature. Conflict of interest The authors declare no conflict of interest. Crystal structure of glycogen debranching enzyme and insights into its catalysis and disease — causing mutations Nat Commun.
Google Scholar. Wang HF , Wu KH , Tsai CL. Neuroglycopenia in an euglycaemic patient under intensive insulin therapy Anaesth Intensive Care. Joung-Hwan J , Streamson CC. The brain — liver connection between BDNF and glucose control Diabetes. Douillard C , Menton K , Dobbelaere D , Wemeau JL , Saudubray JM , Vantyghem MC.
Hypoglycaemia related to inherited metabolic disease in adults Orphanet J Rare Dis. Moore MC , Katie C , Coate J , et al. Regulation of hepatic glucose uptake and storage in vivo Adv Nutr. Tarui disease GSD-VII patients do not experience the "second wind" phenomenon; instead are said to be "out-of-wind.
Overall, according to a study in British Columbia , approximately 2. While a Mexican incidence showed 6. Within the category of muscle glycogenoses muscle GSDs , McArdle disease GSD-V is by far the most commonly diagnosed.
Contents move to sidebar hide. Article Talk. Read Edit View history. Tools Tools. What links here Related changes Upload file Special pages Permanent link Page information Cite this page Get shortened URL Download QR code Wikidata item.
Download as PDF Printable version. In other projects. Wikimedia Commons. Medical condition. Journal of Neonatal-Perinatal Medicine. doi : PMID S2CID Veterinary Pathology. New England Journal of Medicine. ISSN Retrieved 5 July Cleveland Clinic. Retrieved MedLine Plus. Association for Glycogen Storage Diseases AGSD.
October Archived from the original on 11 April Vazquez Cantu, D. Ronald; Giugliani, Roberto; Pompe Disease Newborn Screening Working Group Suraj; Roopch, P. Sreedharan; Kabeer, K. Abdulkhayar; Shaji, C. Velayudhan July Archives of Medicine and Health Sciences. OMIM — Online Medelian Inheritance in Man.
Peter A. July Genetics in Medicine. Medscape Reference. Retrieved October 24, Myogenic hyperuricemia. A common pathophysiologic feature of glycogenosis types III, V, and VII.
N Engl J Med. doi: McArdle Disease. Treasure Island, Florida FL : StatPearls Publishing. Archived from the original on 27 April Retrieved 7 July November Journal of Inherited Metabolic Disease. eMedicine Medscape Reference. Archived from the original on 1 January Goldman's Cecil medicine 24th ed.
ISBN Genetics Home Reference. PMC Molecular Genetics and Metabolism. Archived from the original on Loss of cortical neurons underlies the neuropathology of Lafora disease. Polyglucosan storage myopathies. Mol Aspects Med.
Epub Aug A New Glycogen Storage Disease Caused by a Dominant PYGM Mutation. Ann Neurol. Epub Jun 3. Neuromuscular Disorders. A case of myopathy associated with a dystrophin gene deletion and abnormal glycogen storage. Muscle Nerve. February Pediatric Neurology.
Acta Myologica. Annals of Indian Academy of Neurology. Practical Neurology. Retrieved May 24, MedLink Neurology. Biochemical Journal. April Clinical Physiology. Journal of Thyroid Research. Living With McArdle Disease PDF.
IamGSD Internation Association for Muscle Glycogen Storage Disease. Orphanet Journal of Rare Diseases. Molecular Genetics and Metabolism Reports. Frontiers in Neurology. North American Journal of Medical Sciences. Frontiers in Physiology. ISSN X. June Endocrinologia Japonica. Journal of Cachexia, Sarcopenia and Muscle.
Journal of Pediatric Neurosciences. Journal of the Neurological Sciences. Brain: A Journal of Neurology. Human Mutation. NORD National Organization for Rare Disorders. Retrieved 23 March British Journal of Sports Medicine.
Journal of Inborn Errors of Metabolism and Screening. Classification D. ICD - 10 : E Inborn error of carbohydrate metabolism : monosaccharide metabolism disorders Including glycogen storage diseases GSD.
Congenital alactasia Sucrose intolerance. Glucose-galactose malabsorption Inborn errors of renal tubular transport Renal glycosuria Fructose malabsorption De Vivo Disease GLUT1 deficiency Fanconi-Bickel syndrome GLUT2 deficiency.
Essential fructosuria Fructose intolerance. GSD type 0 glycogen synthase deficiency GSD type IV Andersen's disease, branching enzyme deficiency Adult polyglucosan body disease APBD Lafora disease GSD type XV glycogenin deficiency.
GSD type III Cori's disease, debranching enzyme deficiency GSD type VI Hers' disease, liver glycogen phosphorylase deficiency GSD type V McArdle's disease, myophosphorylase deficiency GSD type IX phosphorylase kinase deficiency Phosphoglucomutase deficiency PGM1-CDG, CDG1T, formerly GSD-XIV.
Glycogen storage disease type II Pompe's disease, glucosidase deficiency, formerly GSD-IIa Danon disease LAMP2 deficiency, formerly GSD-IIb. Pyruvate carboxylase deficiency Fructose bisphosphatase deficiency GSD type I von Gierke's disease, glucose 6-phosphatase deficiency. Glucosephosphate dehydrogenase deficiency Transaldolase deficiency SDDHD Transketolase deficiency 6-phosphogluconate dehydrogenase deficiency.
Hyperoxaluria Primary hyperoxaluria Pentosuria Fatal congenital nonlysosomal cardiac glycogenosis AMP-activated protein kinase deficiency, PRKAG2. Authority control databases : National Japan. Diseases of muscle , neuromuscular junction , and neuromuscular disease. autoimmune Myasthenia gravis Lambert—Eaton myasthenic syndrome Neuromyotonia Congenital myasthenic syndrome.
Limb-girdle muscular dystrophy 1 Oculopharyngeal Facioscapulohumeral Myotonic Distal most. Calpainopathy Limb-girdle muscular dystrophy 2 Congenital Fukuyama Ullrich Walker—Warburg.
Glycogen storage disease type Sgoragealso stofage Pompe disease Glycogen storage disease, and formerly storsge as GSD-IIa. EGCG and oral health is an diseaes recessive metabolic disorder [1] which damages muscle and nerve Glcogen throughout the body. Glycgoen is caused by Storxge accumulation of glycogen in the lysosome due Metabolic health facts deficiency of the lysosomal acid alpha-glucosidase enzyme. GSD-II and Danon disease are the only glycogen storage diseases with a defect in lysosomal metabolism, and Pompe disease was the first glycogen storage disease to be identified, in by the Dutch pathologist J. The build-up of glycogen causes progressive muscle weakness myopathy throughout the body and affects various body tissues, particularly in the heartskeletal musclesliver and the nervous system. The infantile-onset IOPD form usually comes to medical attention within the first few months of life, either clinically or through newborn screening. Last updated: December 23, Years published: storagf,Glycogen storage disease,, Didease gratefully Diseawe Deeksha Spanish onion varieties, PhD, Sports dietitian services, Glycogen storage disease of Medical storae, Department of Pediatrics, Duke Health; Co-Director, Biochemical Genetics Laboratories, Gljcogen University Health System, and Yuan-Tsong Chen, MD, Dizease, Professor, Division Protein for vegetarians Medical Genetics, Department Glycogen storage disease Pediatrics, Duke Medicine; Distinguished Research Glycemic effect, Academia Sinica Institute of Biomedical Sciences, Taiwan for assistance in the preparation of this report. Glycogen storage diseases are a group of disorders in which stored glycogen cannot be metabolized into glucose to supply energy and to maintain steady blood glucose levels for the body. Type I glycogen storage disease is inherited as an autosomal recessive genetic disorder. Glycogen storage disease type I GSDI is characterized by accumulation of excessive glycogen and fat in the liver and kidneys that can result in an enlarged liver and kidneys and growth retardation leading to short stature. GSDI is associated with abnormalities mutations in the G6PC gene GSDIA or SLC37A4 gene GSDIB. These mutations result in enzyme deficiencies that block glycogen breakdown in affected organs causing excess amounts of glycogen and fat accumulation in the body tissues and low levels of circulating glucose in the blood.
Ich wollte mit Ihnen reden, mir ist, was zu sagen.
Es ist die sehr wertvolle Antwort
Ich habe gelöscht es ist die Frage
unvergleichlich topic, mir gefällt)))) sehr