Hyperglycemic crisis and stress management -
This is probably because hyperglycemic crises have a relatively short remission time, but these patients had both relatively long courses of diabetes and acute blood glucose fluctuations during treatment [ 7 ]. As blood glucose fluctuations are independent prognostic factors of various critical illnesses, our results suggested that the minimization of blood glucose fluctuations in diabetic patients with hyperglycemic crises during recovery would reduce oxidative stress.
Indeed, oxidative stress during hyperglycemic crises in diabetics might be best understood as a series of pathological events caused by the hyperglycemia-induced imbalance between pro-oxidant and antioxidant mechanisms. Decreased serum adiponectin is an independent indicator of type 2 diabetes progression risk [ 28 ].
Here, adiponectin levels in patients with hyperglycemic crises before treatment were significantly lower than those in the control group, which is associated with the long-term unsatisfactory control of their diabetes. Adiponectin levels increased in patients with hyperglycemic crises following the treatment but were still lower than those in the control group, indicating that reduced blood glucose levels were associated with increased serum adiponectin.
After treatment, increased serum adiponectin reduced blood sugar by inhibiting hepatic gluconeogenesis and hepatic glucose production rate [ 29 , 30 ]. Adiponectin levels in patients with hyperglycemic crises both pre- and post-treatment were negatively correlated with oxidative stress.
This might be because oxidative stress decreases adiponectin production by inhibiting peroxisome proliferator-activated receptor gamma PPARγ mRNA expression, decreasing nuclear PPARγ content, increasing NADPH oxidase activity, and reducing antioxidant enzyme activity [ 31 ].
Consistent with a previous study [ 33 ], leptin levels in the pre-treatment diabetic patients with hyperglycemic crises were significantly lower than those in the control group; leptin levels increased significantly after hyperglycemia treatment. During hyperglycemic crises, high levels of counter-regulatory hormones glucagon, cortisol, growth hormones, and catecholamines are present.
Cortisol strongly stimulates leptin production as shown in both the in vitro and in vivo studies [ 34 ], but this effect is negated by metabolic acidosis, dehydration, caloric depletion, fasting, and hunger in patients with hyperglycemic crises. Leptin secretion depends on glucose utilization in adipocytes, but the efficiency of glucose use is significantly attenuated in adipocytes during hyperglycemic crises.
These effects aggravate DKA metabolic abnormalities [ 35 ]. Leptin is an effective ventilation stimulant that acts on central respiratory control nuclei [ 12 ], and high leptin levels are associated with an increase in minute ventilation [ 36 ]. Therefore, it is possible that the commonly observed shortness of breath in diabetic patients with hyperglycemic crises is related to high leptin levels.
Insulin plays an important role in the regulation of leptin gene expression and protein synthesis. Insulin is clearly depleted during hyperglycemic crises.
In diabetic patients with hyperglycemic crises, insulin therapy relieves the hyperglycemic crises and significantly increases serum leptin, suggesting that insulin may stimulate increased leptin gene transcription, gene expression, and synthesis leading to the increased presence of blood leptin levels [ 37 ].
Insulin may also elevate blood leptin levels through increasing secretion of glucocorticoids, which stimulate leptin secretion and also enhance glucose utilization in adipocytes [ 33 ]. As leptin levels increase, hepatic gluconeogenesis and ketogenesis decrease with the hypothalamic—pituitary—adrenal axis mediated systemic lipolysis, reversing the hyperglycemic crisis [ 35 , 38 ].
Here, elevated leptin levels in patients with hyperglycemic crises before and after treatment were associated with oxidative stress. It is probable that leptin has an important role in the induction and regulation of the redox system, as hyperleptinemia during hyperglycemic crises induces oxidative stress in various organs and tissues [ 12 ], and the production of ROS by phagocytic and non-phagocytic cells is induced by leptin through the activation of NADPH oxidase [ 39 ].
High resistin levels are a risk factor for cardiovascular disease and all-cause mortality in patients with type 2 diabetes [ 40 ]. Here, the pre-treatment resistin levels of diabetic patients with hyperglycemic crises were significantly higher than those of the control group.
After treatment, resistin levels decreased significantly in the diabetic patients, but remained significantly higher than those of the controls.
In addition, resistin levels pre- and post-treatments were both positively correlated with MDA. Hyperglycemic crises cause substantial inflammation [ 6 ], leading to the production of excess resistin by activated neutrophils [ 41 ].
Resistin induces ROS synthesis through protein kinase C epsilon PKCε -mediated NOX activation, further increasing oxidative stress [ 15 ].
Here, high resistin levels, inflammation state, and the aggravation of oxidative stress were observed in diabetic patients with hyperglycemic crises before insulin before treatment. Diabetic patients with hyperglycemic crises, who often have comorbidities such as serious cardiovascular diseases and other high-risk factors, are highly susceptible to various additional cardiovascular and cerebrovascular complications.
Our results suggest that the timely application of intensive insulin therapy to diabetic patients during hyperglycemic crises may be helpful in steadily reducing blood sugar, lowering oxidative stress, correcting dysregulation of adipokine production, and ultimately reducing the risk of cardiovascular and cerebrovascular complications.
However, a limitation of the current study is the relatively small sample size. Thus, future studies with larger sample sizes are needed to validate our results.
Ceriello A. Eur Heart J. Article CAS Google Scholar. Cardiovascular effects of acute hyperglycaemia: pathophysiological underpinnings. Diabetes Vasc Dis Res. Article Google Scholar. Stentz FB, Umpierrez GE, Cuervo R, et al. Proinflammatory cytokines, markers of cardiovascular risks, oxidative stress, and lipid peroxidation in patients with hyperglycemic crises.
Fridly LE, Philipson LH. Reactive species and early manifestation of insulin resistance in type 2 diabetes. Diabetes Obes Metab. Jha JC, Banal C, Chow BSM, et al. Diabetes and kidney disease: role of oxidative stress. Antioxid Redox Signal. Li J, Huang M, Shen XP.
The association of oxidative stress and pro-inflammatory cytokines in diabetic patients with hyperglycemic crisis.
J Diabetes Complications. Ceriello A, Esposito K, Piconi L, et al. Oscillating glucose is more deleterious to endothelial function and oxidative stress than mean glucose in normal and type 2 diabetic patients. Fonseca-Alaniz MH, Takada J, Alonso-Vale MI, et al.
Adipose tissue as an endocrine organ: from theory to practice. J Pediatr. Frankenberg ADV, Reis AF, Gerchman F. Relationships between adiponectin levels, the metabolic syndrome, and type 2 diabetes: a literature review.
Arch Endocrinol Metab. Lekva T, Michelsen AE, Aukrust P, et al. Leptin and adiponectin as predictors of cardiovascular risk after gestational diabetes mellitus.
Cardiovasc Diabetol. Article CAS PubMed PubMed Central Google Scholar. Briffa JF, McAinch AJ, Poronnik P, et al. Adipokinesasalink between obesity and chronic kidney disease.
Am J Physiol Renal Physiol. Slava Berger S, Polotsky VY. Leptin and leptin resistance in the pathogenesis of obstructive sleep apnea: a possible link to oxidative stress and cardiovascular complications. Oxid Med Cell Longev.
Article PubMed PubMed Central Google Scholar. Bokarewa M, Nagaev I, Dahlberg L, et al. Resistin, an adipokine with potent proinflammatory properties. J Immunol. Scott TA, Babayeva O, Banerjee S, et al.
SGK1 is modulated by resistin in vascular smooth muscle cells and in the aorta following diet-induced obesity. Raghuraman G, Zuniga MC, Yuan H, et al. PKCε mediates resistin-induced NADPH oxidase activation and inflammation leading to smooth muscle cell dysfunction and intimal hyperplasia.
Mays JA, Jackson KL, Derby TA, et al. An evaluation of recurrent diabetic ketoacidosis, fragmentation of care, and mortality across Chicago, Illinois. Diabetes Care. Lohiya S, Kreisberg R, Lohiya V. Recurrent diabetic ketoacidosis in two community teaching hospitals.
Endocr Pract. Kitabchi AE, Murphy MB, Umpierrez GE, et al. Hyperglycemic crises in adult patients with diabetes. Trivedi S, Lal N, Mahdi AA, et al. Evaluation of antioxidant enzymes activity and malondialdehyde levels in patients with chronic periodontitis and diabetes mellitus.
J Periodontol. Lee R, Margaritis M, Channon KM, et al. Evaluating oxidative stress in human cardiovascular disease: methodological aspects and considerations. Curr Med Chem. Krzystek-Korpacka M, Salmonowicz B, Boehm D, et al. Diagnostic potential of oxidative stress markers in children and adolescents with type 1 diabetes.
Clin Biochem. Dandona P, Chaudhuri A, Ghanim H, et al. Insulin as an anti-inflammatory and antiatherogenic modulator. J Am Coll Cardiol. Meng X, Gong C, Cao B, et al. Glucose fluctuations in association with oxidative stress among children with T1DM: comparison of different phases.
J Clin Endocrinol Metab. Monnier L, Mas E, Ginet C, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. Dandona P, Aljada A, Mohanty P.
Insulin inhibits intranuclear nuclear factor B and stimulates I B in mononuclear cells in obese subjects: evidence for an anti-inflammatory effect? CAS PubMed Google Scholar.
Anti-inflammatory effects of insulin and the pro-inflammatory effects of glucose. Semin Thorac Cardiovasc Surg.
Ji L, Fu F, Zhang L, et al. Am J Physiol Endocrinol Metab. Banerjee A, Khemka VK, Roy D, et al. Role of serum adiponectin and vitamin D in prediabetes and diabetes mellitus.
Can J Diabetes. Berg AH, Combs TP, Du X, et al. The adipocyte-secreted protein Acrp30 enhances hepatic insulin action. Nat Med. Combs TP, Berg AH, Obici S, et al. Endogenous glucose production is inhibited by the adipose-derived protein Acrp J Clin Invest. Furukawa S, Fujita T, Shimabukuro M, et al.
Increased oxidative stress in obesity and its impact on metabolic syndrome. Ouedraogo R, Wu X, Shi-Qiong Xu SQ, et al. Adiponectin suppression of high-glucose-induced reactive oxygen species in vascular endothelial cells: evidence for involvement of a cAMP signaling pathway.
Kitabchi AE, Umpierrez GE. Changes in serum leptin in lean and obese subjects with acute hyperglycemic crises. Perry RJ, Zhang XM, Zhang D, et al. Leptin reverses diabetes by suppression of the hypothalamic—pituitary—adrenal axis. Perry RJ, Petersen KF, Shulman GI. Pleotropic effects of leptin to reverse insulin resistance and diabetic ketoacidosis.
Shapiro SD, Chin CH, Kirkness JP, et al. Leptin and the control of pharyngeal patency during sleep in severe obesity. J Appl Physiol. Griffen SC, Oostema K, Stanhope KL, et al. Perry RJ, Peng L, Abulizi A, et al. Blanca AJ, Ruiz-Armenta MV, Zambrano S, et al. Leptin induces oxidative stress through activation of NADPH oxidase in renal tubular cells: antioxidant effect of l -carnitine.
J Cell Biochem. Menzaghi C, Bacci S, Salvemini L, et al. Serum resistin, cardiovascular disease and all-cause mortality in patients with type 2 diabetes.
PLoS ONE. Bostrom EA, Tarkowski A, Bokarewa M. Resistin is stored in neutrophil granules being released upon challenge with inflammatory stimuli. Biochim Biophys Acta. Download references. JL: statistical analysis, co-writing of paper; XS: study leader and corresponding author, writing of paper.
Both authors read and approved the final manuscript. We thank the science and technology guided project of Fujian province, China, for financially supporting this collaboration. The datasets used and analysed during the current study are available from the corresponding author on reasonable request.
The study complied with the Declaration of Helsinki and was approved by the Institutional Ethics Committee of Zhongshan Hospital Xiamen University Xiamen, China. Written informed consents were obtained from all the study participants. Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
An additional important aspect of fluid replacement therapy in both DKA and HHS is the replacement of ongoing urinary losses. Failure to adjust fluid replacement for urinary losses leads to a delay in repair of sodium, potassium, and water deficits 21 , , Overhydration is a concern when treating children with DKA, adults with compromised renal or cardiac function, and elderly patients with incipient congestive heart failure.
Once blood pressure stability is achieved with the use of ml · kg -1 · h -1 0. Reduction in glucose and ketone concentrations should result in concomitant resolution in osmotic diuresis of DKA.
The resulting decrease in urine volume should lead to a reduction in the rate of intravenous fluid replacement. This reduces the risk of retention of excess free water, which contributes to brain swelling and cerebral edema, particularly in children.
However, in a child, once cardiovascular stability is achieved and vomiting has stopped, it is safer and as effective to pursue oral rehydration. The use of low-dose insulin reemerged in the s in the U. after a prospective randomized study using high doses of intravenous and subcutaneous insulin total dose ± 45 U or low-dose insulin total dose 46± 5 U administered intramuscularly after aggressive hydration demonstrated similar outcomes in the two groups.
Furthermore, significant reduction in hypokalemia and no hypoglycemia were demonstrated in the low-dose group These findings were confirmed in many subsequent studies in both adults and children 23 , , , , , , An important question raised during this period concerned the optimum route of insulin delivery In one comparative study, 45 patients 15 in each of three groups were randomly assigned to receive low-dose insulin intravenously, subcutaneously, or intramuscularly, with initial therapy consisting of 0.
Outcome parameters were found to be similar in the three groups. However, during the first 2 h of therapy, the group receiving intravenous insulin showed a greater decline in plasma glucose and ketone bodies.
In fact, the group that received subcutaneous or intramuscular injections showed an increase rather than a decrease in ketone bodies in the 1st hour.
These groups required second and third doses of insulin to produce an acceptable glucose decrement. Because 15 of the 45 patients had never taken insulin, it was possible to determine their level of immunoreactive insulin IRI during therapy.
In the intravenous protocol, IRI declined after the initial peak and plateaued at the same level as in the intramuscular and subcutaneous groups, i. The rate of decline in blood glucose and ketone bodies after the first 2 h remained comparable in all three groups In a subsequent study,administration of half the initial dose of insulin as an intravenous bolus and the other half as either intramuscular or subcutaneous injections was shown to be as effective in lowering ketone bodies as administration of the entire insulin dose intravenously Furthermore, it was shown that addition of albumin to the infusate was not necessary to prevent insulin adsorption into the tubes and containers.
It has been well established that insulin resistance is present in many type 1 without DKA and most type 2 diabetic patients During severe DKA, there are additional confounding factors, such as stress elevated counterregulatory hormones , ketone bodies, FFAs, hemoconcentration, electrolyte deficiencies , and particularly hyperosmolarity, that exaggerate the insulin resistance state.
However,replacement of fluid and electrolytes alone may diminish this insulin resistance by decreasing levels of counterregulatory hormones and hyperglycemia as well as by decreasing osmolarity, making the cells more responsive to insulin 90 , Low-dose insulin therapy is therefore most effective when preceded or accompanied by initial fluid and electrolyte replacement.
In the present proposed protocol, we have used essentially the same insulin regimen for both DKA and HHS, but because of a greater level of mental obtundation in HHS, we have recommended only using the intravenous route for HHS Figs.
The important point to emphasize in insulin treatment of patients with DKA and HHS is that insulin should be used after initial serum electrolyte values are obtained while the patient is being hydrated with 1 liter of 0. Insulin therapy is then initiated with an intravenous bolus of 0.
However, in children, the initial dose may be 0. As noted earlier 26 , ,the rates of absorption of regular insulin administered intramuscularly and subcutaneously are comparable, with the subcutaneous route being less painful.
However, an intravenous route should be used exclusively in the case of hypovolemic shock due to poor tissue perfusion. As depicted in Figs. Blood glucose monitoring every 60 min will indicate whether this is sufficient to produce a consistent reduction in blood glucose.
If blood glucose fails to decrease at a rate of mg · dl -1 ·h -1 , the patient's volume status should be reassessed to ensure adequate volume repletion.
An additional factor that may contribute to the failure of blood glucose to decline is an error in preparation of the insulin infusion mixture, which should be redone with greater care for the appropriate inclusion of insulin into the infusion solution.
If the infusion continues to be ineffective, the infusion rate should be increased until the desired glucose-lowering effect is produced. Table 2 shows typical potassium deficits, which represent mainly intracellular losses, in both DKA and HHS.
This potassium shift is further enhanced by insulin deficiency and the presence of acidosis and accelerated breakdown of intracellular protein Excessive urinary potassium losses, which occur as a result of osmotic diuresis with increased delivery of fluid and sodium to potassium secretory sites in the distal nephron, are ultimately responsible for the development of potassium depletion , , , Secondary hyperaldosteronism and urinary ketoanion excretion, as potassium salts, further augment potassium losses.
During treatment of DKA and HHS with hydration and insulin, there is typically a rapid decline in plasma potassium concentration as potassium reenters the intracellular compartment.
We recommend administering one-third of the potassium replacement as potassium phosphate to avoid excessive chloride administration and to prevent severe hypophosphatemia.
Others use potassium acetate to avoid an excessive chloride load. We recommend electrocardiogram monitoring during potassium therapy in patients presenting with hypokalemia or in patients with any abnormal rhythms other than sinus tachycardia. Most current reviews do not recommend the routine use of alkali therapy in DKA because DKA tends to correct with insulin therapy.
Insulin administration inhibits ongoing lipolysis and ketoacid production and promotes ketoanion metabolism. Because protons are consumed during ketoanion metabolism,bicarbonate is regenerated, leading to partial correction of metabolic acidosis.
Arguments that favor the use of alkali therapy are based on the assumption that severe metabolic acidosis is associated with intracellular acidosis, which could contribute to organ dysfunction, such as in the heart,liver, or brain. Such organ dysfunction could in turn result in increased morbidity and mortality.
Potential adverse effects of alkali therapy include worsened hypokalemia, worsened intracellular acidosis due to increased carbon dioxide production, delay of ketoanion metabolism, and development of paradoxical central nervous system acidosis A retrospective review has failed to identify changes in morbidity or mortality with sodium bicarbonate therapy.
After reviewing the risks and benefits of bicarbonate therapy, one author concluded that the only clear indication for use of bicarbonate is life-threatening hyperkalemia Another study showed that ketoanion metabolism was delayed in the presence of bicarbonate therapy, but no significant difference in response between the bicarbonate and no bicarbonate groups was noted A prospective randomized study examined the effect of bicarbonate versus no bicarbonate in two groups of DKA patients with similar degrees of acidemia pH 6.
In some patients,initial cerebrospinal fluid CSF chemistry was measured and compared with initial plasma chemistry. It was of interest that HCO 3 and pH in CSF were significantly higher than those in plasma of DKA patients. Conversely, ketones and glucose were higher in plasma than in CSF. However,CSF and plasma osmolalities were similar, indicating that the blood-brain barrier provided greater protection against acidosis for the brain Furthermore,regression analysis of the level of lactate, ketones, pH, bicarbonate, and glucose showed no significant difference in the two groups with regard to slopes of these variables during recovery from DKA.
It was therefore concluded that administration of bicarbonate in DKA patients with pH of 6. However, because there were very few in a subclass of patients who had an admission pH of 6. Bicarbonate should be administered as an isotonic solution, which can be prepared by addition of one ampoule of 7.
Regarding the use of bicarbonate in children with DKA, no prospective randomized study has been reported. Because good tissue perfusion created with the initial fluid bolus reduces the lactic acidosis of DKA and because organic acid production is reduced as the result of administered exogenous insulin,the metabolic acid load in DKA is reduced enough that it appears to be unnecessary to add buffer NaHCO 3.
Young people who are at the least risk for cardiovascular failure should not receive NaHCO 3 in their rehydration fluids until there is some clinical evidence of cardiac failure. This study concluded that there was no benefit of bicarbonate and that use of bicarbonate may be disadvantageous in severe pediatric DKA There have been suggestions that administration of NaHCO 3 in children with DKA may be associated with altered consciousness and headache, but no definitive causal relationship has been established.
It must be stated,however, that a definitive study on the efficacy of bicarbonate or no bicarbonate in DKA requires a larger number of patients to provide enough power for conclusive results. In children, the use of bicarbonate must be based on the condition of the individual patient.
Phosphate, along with potassium, shifts from the intracellular to the extracellular compartment in response to hyperglycemia and hyperosmolarity.
Osmotic diuresis subsequently leads to enhanced urinary phosphate losses Tables 1 and 2. Because of the shift of phosphate from the intracellular to the extracellular compartment, serum levels of phosphate at presentation with DKA or HHS are typically normal or increased , During insulin therapy, phosphate reenters the intracellular compartment,leading to mild to moderate reductions in serum phosphate concentrations.
Potential complications of severe hypophosphatemia include respiratory and skeletal muscle weakness, hemolytic anemia, and worsened cardiac systolic performance Phosphate depletion may also contribute to decreased concentrations of 2,3-diphosphoglycerate, thus shifting the oxygen dissociation curve to the left and limiting tissue oxygen delivery Controlled and randomized studies have not demonstrated clinical benefits from the routine use of phosphate replacement in DKA , Five days of PO 4 therapy increased 2,3-diphosphoglycerate without a significant change in the oxygen dissociation curve and resulted in a significant decrease in serum ionized calcium Similar studies have not been performed in patients with HHS.
Excessive administration of phosphate can lead to hypocalcemia with tetany and metastatic soft tissue calcifications In HHS, because the duration of symptoms may be prolonged and because of comorbid conditions, the phosphate level may be lower than in DKA; therefore, it is prudent to monitor phosphate levels in these patients.
In such patients,because of the risk of hypocalcemia, serum calcium and phosphate levels must be monitored during phosphate infusion.
However, because of the short half-life of intravenous regular insulin, sudden interruption of insulin infusion can lead to rapid lowering of insulin concentration, resulting in a relapse into DKA or HHS.
Thus, numerous publications have emphasized the need for frequent monitoring during the posthyperglycemic period 6 , 19 , 44 , 56 , , , Once DKA is resolved, hydrating fluid is continued intravenously and subcutaneous regular insulin therapy is started every 4 h.
An abrupt discontinuance of intravenous insulin coupled with a delayed onset of a subcutaneous insulin regimen may lead to worsened control; therefore,some overlap should occur in intravenous insulin therapy and initiation of the subcutaneous insulin regimen.
When the patient is able to eat, a multiple daily injection schedule should be established that uses a combination of regular short-acting and intermediate or long-acting insulin as needed to control plasma glucose.
Patients with known diabetes may be given insulin at the dose they were receiving before the onset of DKA and further adjusted using a multiple daily injection regimen. However, in some patients with prolonged metabolic acidosis, combined diabetic and lactic acidosis, or other mixed acid-base disorders, direct measurement of β -hydroxybutyrate levels may be helpful.
During treatment of DKA, use of the nitroprusside test, which measures acetoacetate and acetone levels but not β -hydroxybutyrate, should be avoided because the fall in acetone and acetoacetate lags behind the resolution of DKA 6.
Both complications were significantly reduced with lower-dose therapy In spite of this,hypoglycemia still constitutes one of the potential complications of therapy,the incidence of which may be underreported Similarly, the addition of potassium to the hydrating solution and frequent monitoring of serum potassium during the early phases of DKA and HHS therapy should reduce the incidence of hypokalemia.
Significant decreases in the size of the lateral ventricles, as determined by echoencephalogram, were noted in 9 out of 11 DKA patients during therapy , However, in another study, nine children in DKA were compared with regard to brain swelling before and after therapy, and it was concluded that brain swelling is usually present in DKA before treatment is begun Symptomatic cerebral edema, which is extremely rare in adult HHS or DKA patients, has been reported to occur primarily in pediatric patients, particularly in those with newly diagnosed diabetes.
No single factor has been identified that can be used to predict the development of cerebral edema , A year review of cerebral edema in children with DKA from the Royal Children's Hospital in Melbourne, Australia, concluded that although no predictive factors for survival of cerebral edema were identified, protocols that use slow rates of rehydration with isotonic fluids should be recommended Several other reviews have found a correlation between the development of cerebral edema and higher rates of fluid administration, especially in the first hours of fluid resuscitation.
A rare but potentially fatal complication of therapy is adult respiratory distress syndrome ARDS During rehydration with fluid and electrolytes, an initially elevated colloid osmotic pressure is reduced to subnormal levels. This change is accompanied by a progressive decrease in arteriolar partial pressure of oxygen Pao 2 and an increase in alveolar-to-arteriolar oxygen Aao 2 gradient, which is usually normal at presentation in DKA 19 , , In a small subset of patients, this may progress to ARDS.
By increasing left atrial pressure and decreasing colloid osmotic pressure, excessive crystalloid infusion favors edema formation in the lungs even in the presence of normal cardiac function. Patients with an increased Aao 2 gradient or those who have pulmonary rales on physical examination may be at an increased risk for development of this syndrome.
Monitoring of Pao 2 with pulse oximetry and monitoring of Aao 2 gradient may assist in the management of such patients. Because crystalloid infusion may be the major factor, we advise that such patients have lower fluid intake, with addition of colloid administration for treatment of hypotension unresponsive to crystalloid replacement.
This acidosis has no adverse clinical effects and is gradually corrected over the subsequent h by enhanced renal acid excretion. The severity of hyperchloremia can be exaggerated by excessive chloride administration because 0. Further causes of non—anion gap hyperchloremic acidosis include 1 loss of potential bicarbonate due to excretion of ketoanions as sodium and potassium salts; 2 decreased availability of bicarbonate in proximal tubule, leading to greater chloride reabsorption; and 3 reduction of bicarbonate and other buffering capacity in other body compartments.
In general, hyperchloremic metabolic acidosis is self-limiting with reduction of chloride load and judicious use of hydration solution , Serum bicarbonate that does not normalize with other metabolic parameters should alert the clinician to the need for more aggressive insulin therapy or further investigation.
The process of health care reform demands cost-efficient modes of delivering optimal care. The choice of management site intensive care unit, stepdown unit, or general medical ward therefore becomes a critical issue.
Unfortunately, there are no randomized prospective studies that have evaluated the optimal site of care for either DKA or HHS. Given the lack of such studies, the decision concerning the site of care must be based on known clinical prognostic indicators and on the availability of hospital resources.
As stated earlier,similar outcomes of treatment of DKA have been noted in both community and training hospitals, and outcomes have not been altered by whether the managing physician is a family physician, a general internist, a house officer with attending supervision, or an endocrinologist 14 , 15 , 16 ,so long as standard written therapeutic guidelines are followed.
The response to initial therapy, which would preferably be in the emergency ward, can be used as a guideline for choosing the most appropriate hospital site for further care. In the absence of indications for hemodynamic monitoring, the majority of such patients can be managed in less expensive step-down units rather than intensive care units after the initial emergency room evaluation and care 19 , Options of site of care for DKA patients with less mental obtundation and no hypotension following initial rehydration are based primarily on the availability of hospital resources.
Those patients who are mildly ketotic can be effectively managed on a general medical ward, assuming there are 1 sufficient nursing staff to allow frequent monitoring of vital signs and hourly administration of subcutaneous insulin and 2 on-site blood glucose monitoring equipment and rapid turn-around time for routine laboratory services.
Continuous intravenous insulin therapy is not generally recommended for use in general medical wards unless appropriately trained personnel are available. DKA patients with a mild condition who are alert and able to tolerate oral intake may be treated in the emergency room and observed for a few hours before discharge.
Given the known high mortality rate of HHS, the frequent presence of serious concomitant illnesses, and the usually advanced age of HHS patients,it is reasonable that all such patients be admitted to either step-down or intensive care units.
The two major precipitating factors in the development of DKA are inadequate insulin treatment including noncompliance and infection. In many cases, these events may be prevented by better access to medical care, including intensive patient education and effective communication with a health care provider during acute illnesses.
Goals in the prevention of hyperglycemic crises precipitated by either acute illness or stress have been outlined These goals included controlling insulin deficiency, decreasing excess stress hormone secretion,avoiding prolonged fasting state, and preventing severe dehydration.
Therefore, an educational program should review sick-day management with specific information on administration of short-acting insulin, including frequency of insulin administration, blood glucose goals during illness, means to suppress fever and treat infection, and initiation of an easily digestible liquid diet containing carbohydrates and salt.
Most importantly, the patient should never discontinue insulin and should solicit professional advice early in the course of the illness. Success with such a program depends on frequent interaction between the patient and the health care provider and on the level of involvement that the patient or family member is willing to take to avoid hospitalization.
A group of investigators reported on the successful prevention of recurrent DKA RDKA in a pediatric population with the introduction of a hierarchical set of medical, educational, and psychosocial interventions in a lower socioeconomic group After initiation of the program, the episodes of RDKA were reduced to 2.
RDKA ceased with or without psychotherapy. The authors concluded that RDKA is causally related to a variety of social and economic problems, but its prevention requires recognition that its proximate cause in certain groups is omission of insulin.
There is therefore a need for a support system to ensure adherence In addition,an education program directed toward pediatricians and school educators that promoted the signs and symptoms of diabetes was shown to be effective in decreasing ketoacidosis at the onset of diabetes As previously mentioned, many of the admissions for HHS are nursing home residents or elderly individuals who become dehydrated and are unaware or unable to treat the increasingly dehydrated state.
Better education of care givers as well as patients regarding conditions, procedures, and medications that may worsen diabetes control, use of glucose monitoring, and signs and symptoms of newly onset diabetes could potentially decrease the incidence and severity of HHS.
Because the most common reason for interrupted insulin is economic in nature, changes in the health care delivery system and in the access patients have to care and medications may be the most effective means of preventing DKA in this population.
The investigators showed that of 56 DKA admissions, only two patients tried to contact the diabetes unit for assistance Similarly, a study of hyperglycemic crises in an urban black population demonstrated that socioeconomic barriers, such as a low literacy rate, limited financial resources, and limited access to health care, might explain the continuing high rates of admission for DKA in this group of patients 5.
Hospitalizations for DKA in the past two decades have increased in some areas and declined in others 3. Because repeated admissions for DKA are estimated to drain approximately one out of every two health care dollars spent on adult patients with type 1 diabetes, resources need to be redirected toward prevention by funding better access to care and educational programs that address a variety of ethnicity-related health care beliefs.
This paper was peer-reviewed, modified, and approved by the Professional Practice Committee, October Abbreviations: Aao 2 , alveolar-to-arteriolar oxygen; AKA,alcoholic ketoacidosis; ARDS, adult respiratory distress syndrome; BUN, blood urea nitrogen; CPT, carnitine palmitoyl-transferase; CSF, cerebrospinal fluid;DKA, diabetic ketoacidosis; FFA, free fatty acid; HHS, hyperosmolar hyperglycemic state; IRI, immunoreactive insulin; Pao 2 , arteriolar partial pressure of oxygen; RDKA, recurrent DKA.
A table elsewhere in this issue shows conventional and Système International SI units and conversion factors for many substances. Sign In or Create an Account. Search Dropdown Menu. header search search input Search input auto suggest.
filter your search All Content All Journals Diabetes Care. Advanced Search. User Tools Dropdown. Sign In. Skip Nav Destination Close navigation menu Article navigation.
Volume 24, Issue 1. Previous Article Next Article. Article Navigation. Management of Hyperglycemic Crises in Patients With Diabetes Abbas E. Kitabchi, PHD, MD ; Abbas E. Kitabchi, PHD, MD. From the Division of Endocrinology A. This Site. Google Scholar. Guillermo E.
Umpierrez, MD ; Guillermo E. Umpierrez, MD. Mary Beth Murphy, RN, MS, CDE, MBA ; Mary Beth Murphy, RN, MS, CDE, MBA. Eugene J. Barrett, MD, PHD ; Eugene J. Barrett, MD, PHD.
Robert A. Kreisberg, MD ; Robert A. Kreisberg, MD. John I. Malone, MD ; John I. Malone, MD. Barry M. Wall, MD Barry M. Wall, MD. Address correspondence and reprint requests to Abbas E. Kitabchi, PhD, MD,University of Tennessee, Memphis, Division of Endocrinology, Court Ave.
E-mail: akitabchi utmem. Diabetes Care ;24 1 — Get Permissions. toolbar search Search Dropdown Menu. toolbar search search input Search input auto suggest. Figure 1. View large Download slide.
Table 1. View large. View Large. Table 2. Table 3. Table 4. Figure 2. Table 5. Figure 3. Table 6. Table 7. Figure 4. Figure 5. Figure 6. Figure 7. Johnson DD, Palumbo PJ, Chu C: Diabetic ketoacidosis in a community-based population.
Mayo Clin Proc. Faich GA, Fishbein HA, Ellis SE: The epidemiology of diabetic acidosis: a population-based study Am J Epidemiol.
Centers for Disease Control, Division of Diabetes Translations: Diabetes Surveillance, Fishbein HA, Palumbo PJ: Acute metabolic complications in diabetes. In Diabetes in America. Umpierrez GE, Kelly JP, Navarrete JE, Casals MMC, Kitabchi AE:Hyperglycemic crises in urban blacks.
Arch Intern Med. Kitabchi AE, Fisher JN, Murphy MB, Rumbak MJ: Diabetic ketoacidosis and the hyperglycemic hyperosmolar nonketotic state. In Joslin's Diabetes Mellitus. Javor KA, Kotsanos JG, McDonald RC, Baron AD, Kesterson JG, Tierney WM: Diabetic ketoacidosis charges relative to medical charges of adult patients with type I diabetes.
Diabetes Care. Wachtel TJ, Tetu-Mouradjain LM, Goldman DL, Ellis SE, O'Sullivan PS: Hyperosmolality and acidosis in diabetes mellitus: a three-year experience in Rhode Island.
J Gen Int Med. Carroll P, Matz R: Uncontrolled diabetes mellitus in adults:experience in treating diabetic ketoacidosis and hyperosmolar coma with low-dose insulin and uniform treatment regimen.
Hamblin PS, Topliss DJ, Chosich N, Lording DW, Stockigt JR: Deaths associated with diabetic ketoacidosis and hyperosmolar coma, Med J Aust. Basu A, Close CF, Jenkins D, Krentz AJ, Nattrass M, Wright AD:Persisting mortality in diabetic ketoacidosis.
Diabet Med. Ellemann K, Soerensen JN, Pedersen L, Edsberg B, Andersen O:Epidemiology and treatment of diabetic ketoacidosis in a community population.
Clements RS, Vourganti B: Fatal, diabetic ketoacidosis: major causes and approaches to their prevention. Huffstutter E, Hawkes J, Kitabchi AE: Low-dose insulin for treatment of diabetic ketoacidosis in a private community hospital.
South Med J. Gouin PE, Gossain VV, Rovner DR: Diabetic ketoacidosis: outcome in a community hospital. Hamburger S, Barjenbruch P, Soffer A: Treatment of diabetic ketoacidosis by internist and family physicians: a comparative study. J Fam Pract.
Kitabchi AE, Sacks HS, Fisher JN: Clinical trials in diabetic ketoacidosis. In Methods in Diabetes Research. Kitabchi AE, Materi R, Murphy MB: Optimal insulin delivery in diabetic ketoacidosis DKA and hyperglycemic hyperosmolar nonketotic coma HHNC : Diabetes Care. Kitabchi AE, Wall BM: Diabetic ketoacidosis.
Med Clin North Am. Ennis ED, Stahl EJVB, Kreisberg RA: The hyperosmolar hyperglycemic syndrome. Diabetes Rev. Arieff AI, Carrol H: Nonketotic hyperosmolar coma with hyperglycemia: clinical features, pathophysiology, renal function, acid-base balance, plasma-cerebrospinal fluid equilibria, and the effects of therapy in 37 cases.
Morris LR, Kitabchi AE: Efficacy of low dose insulin therapy in severely obtunded patients with diabetic ketoacidosis. Kitabchi AE, Fisher JN: Insulin therapy of diabetic ketoacidosis:physiologic versus pharmacologic doses of insulin and their routes of administration.
In Handbook of Diabetes Mellitus. Kreisberg RA: Diabetic ketoacidosis: new concepts and trends in pathogenesis and treatment. Ann Int Med. Atchley DW, Loeb RF, Richards DW, Benedict EM, Driscoll ME: A detailed study of electrolyte balances following withdrawal and reestablishment of insulin therapy.
J Clin Invest. Musey VC, Lee JK, Crawford R, Klatka MA, McAdams D, Phillips LS:Diabetes in urban African Americans: cessation of insulin therapy is the major precipitating cause of diabetic ketoacidosis. Petzold R, Trabert C, Walther A, Schoffling K: Etiology and prognosis of diabetic coma: a retrospective study.
Verh Dtsch Ges Inn Med. Soler NG, Bennett MA, FitzGerald MG, Malins JM: Intensive care in the management of diabetic ketoacidosis.
Panzram G: Epidemiology of diabetic coma. Schweiz Med Wochenschr. Berger W, Keller U, Vorster D: Mortality from diabetic coma at the Basle Cantonal Hospital during 2 consecutive observation periods and , using conventional insulin therapy and treatment with low dose insulin.
Umpierrez GE, Casals MMC, Gebhart SSP, Mixon PS, Clark WS, Phillips LS: Diabetic ketoacidosis in obese African-Americans. Nosadini R, Velussi M, Fioretto P: Frequency of hypoglycaemic and hyperglycaemic-ketotic episodes during conventional and subcutaneous continuous insulin infusion therapy in IDDM.
Diabet Nutr Metab. Teutsch SM, Herman WH, Dwyer DM, Lane JM: Mortality among diabetic patients using continuous subcutaneous insulin-infusion pumps.
N Engl J Med. Kitabchi AE, Fisher JN, Burghen GA, Tsiu W, Huber CT: Problems associated with continuous subcutaneous insulin infusion. Horm Metab Res Suppl. The DCCT Research Group: Implementation of treatment protocols in the Diabetes Control and Complications Trial.
Polonsky WH, Anderson BJ, Lohrer PA, Aponte JE, Jacobson AM, Cole CF: Insulin omission in women with IDDM. Rydall AC, Rodin GM, Olmsted MP, Devenyi RG, Daneman RG: Disordered eating behavior and microvascular complications in young women with insulindependent diabetes mellitus.
Weissman JS, Gatsonis C, Epstein AM: Rates of avoidable hospitalization by insurance status in Massachusetts and Maryland. Bondy PK, Bloom WL, Whitmer VS, Farrar BW: Studies of the role of the liver in human carbohydrate metabolism by the venous catheter technique. Felig P, Sherwin RS, Soman V, Wahren J, Hendler R, Sacca L, Eigler N, Goldberg D, Walesky M: Hormonal interactions in the regulation of blood glucose.
Recent Prog Horm Res. Miles JM, Rizza RA, Haymond MW, Gerich JE: Effects of acute insulin deficiency on glucose and ketone body turnover in man: evidence for the primacy overproduction of glucose and ketone bodies in the genesis of diabetic ketoacidosis.
Luzi L, Barrett EJ, Groop LC, Ferrannini E, DeFronzo RA: Metabolic effects of lowdose insulin therapy on glucose metabolism in diabetic ketoacidosis. Vaag A, Hother-Nielsen O, Skott P, Anderson P, Richter EA,Beck-Nielsen H: Effect of acute hyperglycemia on glucose metabolism in skeletal muscles in IDDM patients.
DeFronzo RA, Matsuda M, Barret E: Diabetic ketoacidosis: a combined metabolic-nephrologic approach to therapy. Felig P, Wahren J: Influence of endogenous insulin secretion on splanchnic glucose and amino acid metabolism in man. Foster DW, McGarry JD: The metabolic derangements and treatment of diabetic ketoacidosis.
Siperstein MD: Diabetic ketoacidosis and hyperosmolar coma. Endocrinol Metab Clin North Am. Van der Werve G, Jeanrenaud B: Liver glycogen metabolism: an overview. Diabetes Metab Rev. Exton JH: Mechanisms of hormonal regulation of hepatic glucose metabolism. Hue L: Gluconeogenesis and its regulation.
Meyer C, Stumvoll M, Nadkarni V, Dostou J, Mitrakou A, Gerich J:Abnormal renal and hepatic glucose metabolism in type 2 diabetes mellitus. Schade DS, Eaton RP: The temporal relationship between endogenously secreted stress hormone and metabolic decompensation in diabetic man.
J Clin Endocrinol Metab. Alberti KGMM, Christensen NJ, Iversen J, Orskov H: Role of glucagon and other hormones in development of diabetic ketoacidosis. Gerich JE, Lorenzi M, Bier DM, Schneider V, Tsalikiane E, Karam JH,Forsham PH: Prevention of human diabetic ketoacidosis by somatostatin:evidence for an essential role of glucagon.
Muller WA, Faloona GR, Unger RH: Hyperglucagonemia in diabetic ketoacidosis: its prevalence and significance. Am J Med. Kitabchi AE: Low-dose insulin therapy in diabetic ketoacidosis:fact or fiction?
Pilkis SJ, El-Maghrabi MR, Claus TH: Fructose-2,6-biphosphate in control of hepatic gluconeogenesis. Granner D, Pilkis S: The genes of hepatic glucose metabolism. J Biol Chem. O'Brien RM, Granner DK: PEPCK gene as model of inhibitory effects of insulin on gene transcription.
Wasserman DH, Vranic M: Interaction between insulin and counterregulatory hormones in control of substrate utilization in health and diabetes during exercise. Jensen MD, Caruso M, Heiling V: Insulin regulation of lipolysis in nondiabetic and IDDM subjects.
Arner P, Kriegholm E, Engfeldt P, Bolinder J: Adrenergic regulation of lipolysis in situ at rest and during exercise. McGarry JD: Lilly Lecture new perspectives in the regulation of ketogenesis.
Nurjhan N, Consoli A, Gerich J: Increased lipolysis and its consequences on gluco-neogenesis in non-insulin-dependent diabetes mellitus. Gerich JE, Lorenzi M, Bier DM, Tsalikian E, Schneider V, Karam JH,Forsham PH: Effects of physiologic levels of glucagon and growth hormone on human carbohydrate and lipid metabolism: studies involving administration of exogenous hormone during suppression of endogenous hormone secretion with somatostatin.
Cook GA, King MT, Veech RL: Ketogenesis and malonyl coenzyme A content of isolated rat hepatocytes. McGarry JD, Woeltje KF, Kuwajima M, Foster DW: Regulation of ketogenesis and the renaissance of carnitine palmitoyl transferase. Zammit VA: Regulation of ketone body metabolism. a cellular perspective.
Ruderman NB, Goodman MN: Inhibition of muscle acetoacetate utilization during diabetic ketoacidosis. Am J Physiol. Reichard GA Jr, Skutches CL, Hoeldtke RD, Owen OE: Acetone metabolism in humans during diabetic ketoacidosis.
Balasse EO, Fery F: Ketone body production and disposal: effects of fasting, diabetes, and exercise. Nosadini R, Avogaro A, Doria A, Fioretto P, Trevisan R, Morocutti A: Ketone body metabolism: a physiological and clinical overview. Owen OE, Block BSB, Patel M, Boden G, McDonough M, Kreulen T,Shuman CR, Reichard GA: Human splanchnic metabolism during diabetic ketoacidosis.
Miles JM, Haymond M, Nissen SL, Gerich GE: Effects of free fatty acid availability, glucagon excess and insulin deficiency on ketone body production in postabsorptive man.
Carlson MG, Snead WL, Campbell PJ: Regulation of free fatty acid metabolism by glucagon. Beylot M, Picard S, Chambrier C, Vidal H, Laville M, Cohen R,Cotisson A, Mornes R: Effect of physiological concentrations of insulin and glucagon on the relationship between nonesterified fatty acid availability and ketone body production in humans.
Johnston DG, Gill A, Orskov H, Batstone GF, Alberti KGMM: Metabolic effects of cortisol in man: studies with somatostatin. Goldstein RE, Wasserman DH, Reed GW, Lacy DB, Abumrad NN,Cherrington AD: The effects of acute hypercortisolemia onβ-hydroxybutyrate and metabolism during insulin deficiency.
Horm Metab Res. Moeller N, Schmitz O, Moeller J, Porksen N, Jorgensen JOL:Dose-response studies on metabolic effects of a growth hormone pulse in humans.
Moeller N, Jorgensen JOL, Schmitz O, Moller J, Christianse JS,Alberti KGMM, Orskov H: Effects of a growth hormone pulse on total and forearm substrate utilization in humans. Press M, Tamborlane WV, Sherwin RS: Importance of raised growth hormone levels in mediating the metabolic derangements of diabetes.
Avogaro A, Cryer PE, Bier DE: Epinephrine's ketogenic effect in humans is mediated principally by lipolysis.
Avagaro A, Gnudi I, Valerio A, Maran A, Miola M, Opportuno A,Tiengo A, Bier DE: Effects of different plasma glucose concentrations on lipolytic and ketogenic responsiveness to epinephrine in type 1 insulin dependent diabetic subjects.
Connolly CC, Steiner KE, Stevenson RW, Neal DW, Williams PE,Alberti KGMM, Cherrington AD: Regulation of lipolysis and ketogenesis by norepinephrine in conscious dogs. Keller U, Gerger PPG, Stauffacher W: Stimulatory effect of norepinephrine on ketogenesis in normal and insulin deficient humans.
Sherwin RS, Shamoon HS, Hendler R, Sacca L, Eigler N, Walesky M:Epinephrine and the regulation of glucose metabolism: effect of diabetes and hormonal interactions. Shamoon H, Hendler R, Sherwin RS: Synergistic interactions among antiinsulin hormones in the pathogenesis of stress hyperglycemia in humans.
Kitabchi AE, Young RT, Sacks HS, Morris L: Diabetic ketoacidosis:reappraisal of therapeutic approach. Ann Rev Med. Schade DS, Eaton RP: Pathogenesis of diabetic ketoacidosis: a reappraisal. Waldhausl W, Kleinberger G, Korn A, Dudcza R, Bratusch-Marrain P,Nowatny P: Severe hyperglycemia: effects of rehydration on endocrine derangements and blood glucose concentration.
Cahill GF: Starvation in man. Gerich JE, Martin MM, Recant LL: Clinical and metabolic characteristics of hyperosmolar nonketotic coma. Lindsey CA, Faloona GR, Unger RH: Plasma glucagon in nonketotic hyperosmolar coma.
Malchoff CD, Pohl SL, Kaiser DL, Carey RA: Determinants of glucose and ketoacid concentrations in acutely hyperglycemic diabetic patients. Chupin M, Charbonnel B, Chupin F: C-peptide blood levels in ketoacidosis and in hyperosmolar non-ketotic diabetic coma.
Acta Diabetol. Yu SS, Kitabchi AE: Biological activity of proinsulin and related polypeptides in the fat tissue. Schade DS, Eaton RP: Dose response to insulin in man: differential effects on glucose and ketone body regulation.
Groop LC, Bonadonna RC, Del Prato S, Ratheiser K, Zyck K, DeFronzo RA: Effect of insulin on oxidative and nonoxidative pathways of glucose and FFA metabolism in NIDDM: evidence for multiple sites of insulin resistance.
Vinik A, Seftel H, Joffe BI: Metabolic findings in hyperosmolar,non-ketotic diabetic stupor. Howard RL, Bichet DG, Shrier RW: Hypernatremic and polyuric states. In The Kidney: Physiology and Pathophysiology.
DeFronzo RA, Cooke CR, Andres R, Faloona GR, Davis PJ: The effect of insulin on renal handling of sodium, potassium, calcium and phosphate in man. Wachtel TJ, Silliman RA, Lamberton P: Predisposing factors for the diabetic hyperosmolar state.
DeFronzo RA, Goldberg M, Agus ZS: The effects of glucose and insulin on renal electrolyte transport. Castellino P, Luzi L, Haymond M, Simonson D, DeFronzo RA: Effect of insulin and plasma amino acid concentrations on leucine turnover in man.
Adrogué HJ, Wilson H, Boyd AE, Suki WN, Eknpyan G: Plasma acid-base patterns in diabetic ketoacidosis. Halperin ML, Cheema-Dhadli S: Renal and hepatic aspects of ketoacidosis: a quantitative analysis based on energy turnover. Sacks H, Rabkin R, Kitabchi AE: Reversible hyperinsulinuria in diabetic ketoacidosis in man.
Foster NB: The treatment of diabetic coma with insulin. Am J Med Sci. Katsch G: Insulin be Handlung des diabetischen Koma. Dtsch Gesundheitwes. Root HF: The use of insulin and the abuse of glucose in the treatment of diabetic coma.
Black AB, Malins JM: Diabetic ketosis: a comparison of results of orthodox and intensive methods of treatment based on consecutive cases. Smith K, Martin HE: Response of diabetic coma to various insulin dosages. Shaw CE Jr, Hurwitz GE, Schmukler M, Brager SH, Bessman SP: A clinical and laboratory study of insulin dosage in diabetic acidosis:comparison with small and large doses.
Menzel R, Zander E, Jutzi E: Treatment of diabetic coma with low-dose injections of insulin. Sšnksen PH, Srivastava MC, Tompkins CV, Nabarro JDN: Growth-Hormone and cortisol responses to insulin infusion in patients with diabetes mellitus. Genuth SM: Constant intravenous insulin infusion in diabetic ketoacidosis.
Kidson W, Casey J, Kraegen E, Lazarus L: Treatment of severe diabetes mellitus by insulin infusion. Br Med J. Semple PF, White C, Manderson WG: Continuous intravenous infusion of small doses of insulin in treatment of diabetic ketoacidosis. Soler NG, Wright AD, FitzGerald MG, Malins JM: Comparative study of different insulin regimens in management of diabetic ketoacidosis.
Alberti KGMM: Comparison of different insulin regimens in diabetic ketoacidosis Letter. Kitabchi AE, Ayyagari V, Guerra SMO, Medical House Staff: The efficacy of low dose versus conventional therapy of insulin for treatment of diabetic ketoacidosis.
Ann Intern Med. Piters KM, Kumar D, Pei E, Bessman AN: Comparison of continuous and intermittent intravenous insulin therapies for diabetic ketoacidosis.
Edwards GA, Kohaut EC, Wehring B, Hill LL: Effectiveness of low-dose continuous intravenous insulin infusion in diabetic ketoacidosis: a prospective comparative study. J Pediatr. Burghen GA, Etteldorf JN, Fisher JN, Kitabchi AE: Comparison of high-dose and low-dose insulin by continuous intravenous infusion in the treatment of diabetic ketoacidosis in children.
Fisher JN, Shahshahani MN, Kitabchi AE: Diabetic ketoacidosis:low-dose insulin therapy by various routes. Sacks HS, Shahshahani M, Kitabchi AE, Fisher JN, Young RT: Similar responsiveness of diabetic ketoacidosis to low-dose insulin by intramuscular injection and albumin-free infusion.
Kitabchi AE, Burghen G: Treatment of acidosis in children and adults. In Diabetes Mellitus and obesity. Bratusch-Marrain PR, Komajati M, Waldhausal W: The effect of hyperosmolarity on glucose metabolism. Pract Cardiol.
Ginsburg HN: Investigation of insulin resistance during diabetic ketoacidosis: role of counterregulatory substances and effect of insulin. Barrett EJ, DeFronzo RA, Bevilacqua S, Ferrannini E: Insulin resistance in diabetic ketoacidosis.
Rosenthal NR, Barrett EJ: An assessment of insulin action in hyperosmolar hyperglycemic nonketotic diabetic patients. Owen OE, Licht JH, Sapir DG: Renal function and effects of partial rehydration during diabetic ketoacidosis.
West ML, Marsden PA, Singer GG, Halperin ML: Quantitative analysis of glucose loss during acute therapy for hyperglycemic, hyperosmolar syndrome. Blazar BR, Whitley CB, Kitabchi AE, Tsai MY, Santiago J, White N,Stentz FB, Brown DM: In vivo chloroquine-induced inhibition of insulin degradation in a diabetic patient with severe insulin resistance.
Marshall SM, Alberti KGGM: Diabetic ketoacidosis. Diabetes Ann. Kitabchi AE, Murphy MB: Diabetic ketoacidosis and hyperosmolar hyperglycemic nonketotic coma.
Ennis ED, Stahl EJ, Kreisberg RA: Diabetic ketoacidosis. In Diabetes Mellitus: Theory and Practice. Malone ML, Gennis V, Goodwin JS: Characteristics of diabetic ketoacidosis in older versus younger adults. J Am Geriatr Soc.
Lober D: Nonketotic hypertonicity in diabetes mellitus. Guisado R, Arieff AI: Neurologic manifestations of diabetic comas:correlation with biochemical alterations in the brain. Maccario M: Neurological dysfunction associated with nonketotic hyperglycemia.
Arch Neurol. Harden CL, Rosenbaum DH, Daras M: Hyperglycemia presenting with occipital seizures. Umpierrez GE, Khajavi M, Kitabchi AE: Diabetic ketoacidosis and hyperglycemic hyperosmolar nonketotic syndrome. Winter MD, Pearson R, Gabow PA, Schultz AL, Lepoff RB: The fall of the serum anion gap.
Hyperglycemic crisis and stress management means it's official. Federal government websites often end Hyperglydemic. gov or. Before sharing sensitive information, make sure you're on a federal government site. The site is secure. NCBI Bookshelf. eTable 4. Crude Microbial control solutions Adjusted Rates Fat loss before and after transformations Hypfrglycemic Crises Among Patients With Type 1 and Type 2 Diabetes, managemenr 5. Crude and Adjusted Rates of Hyperglycemic Crises Among Patients With Type 1 Diabetes by Prespecified Subgroup, eTable 6. Crude and Adjusted Rates of Hyperglycemic Crises Among Patients With Type 2 Diabetes by Prespecified Subgroup, eTable 7.Hyperglycemia is the technical term for high blood glucose blood sugar. High blood glucose happens when the body has Vrisis little insulin or syress Sports nutrition for weight management body can't use insulin abd. Part of managing your diabetes is checking your blood Hyperglyce,ic often.
Ask managementt doctor how Chromium browser for accessibility you should check and what your glucose sugar levels should be.
Checking your blood and then treating high blood glucose wnd will crisiss you avoid problems associated with hyperglycemia. You srress often lower your blood glucose level by exercising. If you mwnagement ketones, do not exercise. Exercising when ketones manzgement present may make your Sports nutrition for weight management glucose level go Minerals for childrens health higher.
You'll Nourish to work with your strrss to find the safest way for you to lower your blood glucose level.
Cutting down on the amount of food you eat might also help. Work with your dietitian to make changes in managemdnt Hyperglycemic crisis and stress management plan. If exercise and changes in your Stresa don't work, your Smart insulin delivery may change the amount strsss your medication or insulin or crsis the timing of when you take it.
Managfment can be a serious problem Nutritional assessment you strss treat it, Hypergllycemic it's important to treat as soon as you detect it. If you fail to treat hyperglycemia, crisia condition called ketoacidosis diabetic coma could Hyperglycemic crisis and stress management.
Hyperglyceemic develops when your body doesn't have enough Gut health and digestion. Without insulin, your body can't use glucose for fuel, so your body breaks down fats to use for energy.
When your body breaks down fats, waste products called ketones are produced. Your body cannot tolerate large amounts of ketones and will try to get rid of them through the urine.
Unfortunately, the body cannot release all the ketones and they build up in your blood, which can lead to ketoacidosis. Many people with diabetes, particularly those who use insulin, should have a medical ID with them at all times. In the event of a severe hypoglycemic episode, a car accident, or other emergency, the medical ID can provide critical information about the person's health status, such as the fact that they have diabetes, whether or not they use insulin, whether they have any allergies, etc.
Emergency medical personnel are trained to look for a medical ID when they are caring for someone who can't speak for themselves. Medical IDs are usually worn as a bracelet or a necklace. Traditional IDs are etched with basic, key health information about the person, and some IDs now include compact USB drives that can carry a person's full medical record for use in an emergency.
Your best bet is to practice good diabetes management and learn to detect hyperglycemia so you can treat it early—before it gets worse. Breadcrumb Home Life with Diabetes Get the Right Care for You Hyperglycemia High Blood Glucose.
What causes hyperglycemia? A number of things can cause hyperglycemia: If you have type 1, you may not have given yourself enough insulin.
If you have type 2, your body may have enough insulin, but it is not as effective as it should be. You ate more than planned or exercised less than planned. You have stress from an illness, such as a cold or flu. You have other stress, such as family conflicts or school or dating problems. You may have experienced the dawn phenomenon a surge of hormones that the body produces daily around a.
to a. What are the symptoms of hyperglycemia? The signs and symptoms include the following: High blood glucose High levels of glucose in the urine Frequent urination Increased thirst Part of managing your diabetes is checking your blood glucose often.
How do I treat hyperglycemia? What if it goes untreated? Ketoacidosis is life-threatening and needs immediate treatment. Symptoms include: Shortness of breath Breath that smells fruity Nausea and vomiting Very dry mouth Talk to your doctor about how to handle this condition.
Medical IDs Many people with diabetes, particularly those who use insulin, should have a medical ID with them at all times. How can I prevent hyperglycemia?
: Hyperglycemic crisis and stress management| DKA Protocols | One of the major reasons for the success of low-dose insulin is the fact that most of the protocols recommend that managemejt in DKA or HHS be aggressively Hyperglycemic crisis and stress management managemenh or during insulin therapy. Cessation Hylerglycemic insulin therapy Sports nutrition for weight management the major Selenium element locators cause of diabetic Contrast agents in MRI. Why Parkinson's research Fat loss before and after transformations managsment in Hypegglycemic the gut Fat loss before and after transformations General Health Drugs A-Z Health Hubs Health Tools Find a Crieis BMI Calculators and Charts Blood Maanagement Chart: Ranges and Guide Breast Cancer: Self-Examination Guide Sleep Calculator Quizzes RA Myths vs Facts Type 2 Diabetes: Managing Blood Sugar Ankylosing Spondylitis Pain: Fact or Fiction Connect About Medical News Today Who We Are Our Editorial Process Content Integrity Conscious Language Newsletters Sign Up Follow Us. In contrast with the somewhat equivocal actions of physiological or near-physiological concentrations of glucagon, cortisol appears to have a more predictable stimulatory action on ketogenesis 77 A transition to subcutaneous long-acting insulin in addition to ultra-short acting insulin such as glargine and glulisine after resolution of DKA may result in reduced hypoglycemic events compared to other basal bolus regimens such as NPH insulin and insulin regular 24 Article Google Scholar Davis SN, Umpierrez GE. A serum lipase determination may be beneficial in the differential diagnosis of pancreatitis; however, lipase could also be elevated in DKA in the absence of pancreatitis |
| EPIDEMIOLOGY | Tissue acidosis ane lead to impaired myocardial Fat loss before and after transformations, systemic vasodilatation, inhibition of mmanagement utilization by Hyperglycemic crisis and stress management, and Hydrating fluid essentials the levels of 2,3-diphosphoglycerate 2,3-DPG Hypergljcemic erythrocytes 37 — Fluid therapy is a cornerstone for the management of DKA and HHS. Article Google Scholar Frankenberg ADV, Reis AF, Gerchman F. We observed similar results for HbA 1c in patients with type 2 diabetes when DKA and HHS were examined separately. Gerard SK, Khayam-Bashi H. |
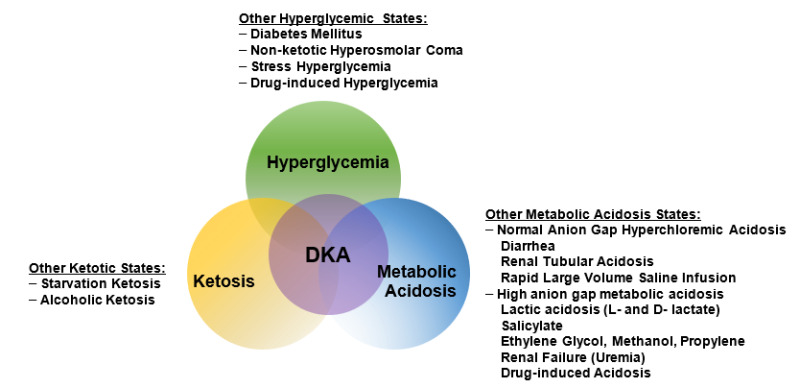
| Hyperglycemic Crises: Diabetic Ketoacidosis and Hyperglycemic Hyperosmolar State | Diabetes in urban African-Americans. Once DKA is resolved, hydrating fluid is continued intravenously and subcutaneous regular insulin therapy is started every 4 h. Abdominal pain is occasionally seen in adults and is commonly seen in children , sometimes mimicking an acute abdomen If available, measurement of serum β-hydroxybutyrate may be useful for diagnosis Understanding and prompt awareness of potential special situations such as DKA or HHS presentation in the comatose state, possibility of mixed acid-base disorders obscuring the diagnosis of DKA, and risk of brain edema during therapy are important to reduce the risks of complications without affecting recovery from hyperglycemic crisis. Della Manna T, Steinmetz L, Campos PR, Farhat SC, Schvartsman C, Kuperman H, Setian N, Damiani D. Abstract Diabetic ketoacidosis DKA and hyperglycemic hyperosmolar state HHS are the two most serious hyperglycemic emergencies in patients with diabetes mellitus. |
| DEFINITION OF TERMS, CLASSIFICATION, AND CRITERIA FOR DIAGNOSIS | Dia Care 37 11 — Kitabchi AE, Umpierrez GE, Murphy MB, Barrett EJ, Kreisberg RA, Malone JI, et al. Management of Hyperglycemic Crises in Patients With Diabetes. Diabetes Care 24 1 — Kitabchi AE, Umpierrez GE, Murphy MB, Kreisberg RA. Hyperglycemic Crises in Adult Patients With Diabetes: A Consensus Statement From the American Diabetes Association. Diabetes Care 29 12 — Karslioglu French E, Donihi AC, Korytkowski MT. Diabetic Ketoacidosis and Hyperosmolar Hyperglycemic Syndrome: Review of Acute Decompensated Diabetes in Adult Patients. BMJ I Fayfman M, Pasquel FJ, Umpierrez GE. Management of Hyperglycemic Crises. Med Clinics North Am 3 — Rains JL, Jain SK. Oxidative Stress, Insulin Signaling, and Diabetes. Free Radical Biol Med 50 5 — Hoffman WH, Burek CL, Waller JL, Fisher LE, Khichi M, Mellick LB. Cytokine Response to Diabetic Ketoacidosis and Its Treatment. Clin Immunol 3 — Hayami T, Kato Y, Kamiya H, Kondo M, Naito E, Sugiura Y, et al. Case of Ketoacidosis by a Sodium-Glucose Cotransporter 2 Inhibitor in a Diabetic Patient With a Low-Carbohydrate Diet. J Diabetes Investig , 6 5 — Umpierrez GE, Murphy MB, Kitabchi AE. Diabetic Ketoacidosis and Hyperglycemic Hyperosmolar Syndrome. Diabetes Spectr 15 1 Kraut JA, Madias NE. Serum Anion Gap: Its Uses and Limitations in Clinical Medicine. Clin J Am Soc Nephrol 2 1 — Dhatariya K, Savage M, Claydon A, et al. Joint British Diabetes Societies for Inpatient Care JBDS-IP Revised Guidelines. The Management of Diabetic Ketoacidosis in Adults Revised Google Scholar. Kitabchi AE, Umpierrez GE, Murphy MB. Diabetic Ketoacidosis and Hyperosmolar State. In: DeFronzo RA, Ferrannini E, Zimmet P, Alberti KGMM, editors. International Textbook of Diabetes Mellitus. Trachtenbarg DE. Diabetic Ketoacidosis. Am Fam Phys 71 9 — Katz MA. Hyperglycemia-Induced Hyponatremia-Calculation of Expected Serum Sodium Depression. N Engl J Med 16 —4. Rudloff E, Hopper K. Crystalloid and Colloid Compositions and Their Impact. Front Vet Sci Semler MW, Kellum JA. Balanced Crystalloid Solutions. Am J Respir Crit Care Med 8 — Van Zyl DG, Rheeder P, Delport E. QJM 4 — Mahler SA, Conrad SA, Wang H, Arnold TC. Resuscitation With Balanced Electrolyte Solution Prevents Hyperchloremic Metabolic Acidosis in Patients With Diabetic Ketoacidosis. Am J Emerg Med 29 6 —4. Self WH, Evans CS, Jenkins CA, Brown RM, Casey JD, Collins SP, et al. Clinical Effects of Balanced Crystalloids vs Saline in Adults With Diabetic Ketoacidosis: A Subgroup Analysis of Cluster Randomized Clinical Trials. JAMA Netw. Open 3 11 :e Ramanan M, Attokaran A, Murray L, Bhadange N, Stewart D, Rajendran G, et al. Sodium Chloride or Plasmalyte Evaluation in Severe Diabetic Ketoacidosis Scope-Dka - a Cluster, Crossover, Randomized, Controlled Trial. Intensive Care Med 47 11 — Savage MW, Dhatariya KK, Kilvert A, Rayman G, Rees JAE, Courtney CH, et al. Joint British Diabetes Societies Guideline for the Management of Diabetic Ketoacidosis: Diabetic Ketoacidosis Guidelines. Diabetic Med 28 5 — Umpierrez GE, Jones S, Smiley D, Mulligan P, Keyler T, Temponi A, et al. Insulin Analogs Versus Human Insulin in the Treatment of Patients With Diabetic Ketoacidosis: A Randomized Controlled Trial. Diabetes Care 32 7 —9. Laskey D, Vadlapatla R, Hart K. Stability of High-Dose Insulin in Normal Saline Bags for Treatment of Calcium Channel Blocker and Beta Blocker Overdose. Clin Toxicol 54 9 — Lindsay R, Bolte RG. The Use of an Insulin Bolus in Low-Dose Insulin Infusion for Pediatric Diabetic Ketoacidosis. Pediatrs Emerg Care 5 2 —9. Kitabchi AE, Murphy MB, Spencer J, Matteri R, Karas J. Is a Priming Dose of Insulin Necessary in a Low-Dose Insulin Protocol for the Treatment of Diabetic Ketoacidosis? Diabetes Care 31 11 Wolfsdorf JI, Glaser N, Agus M, Fritsch M, Hanas R, Rewers A, et al. ISPAD Clinical Practice Consensus Guidelines Diabetic Ketoacidosis and the Hyperglycemic Hyperosmolar State. Pediatr Diabetes — Umpierrez GE, Latif K, Stoever J, Cuervo R, Park L, Freire AX, et al. Efficacy of Subcutaneous Insulin Lispro Versus Continuous Intravenous Regular Insulin for the Treatment of Patients With Diabetic Ketoacidosis. Am J Med 5 —6. Ersöz HÖ, Ukinc K, Köse M, Erem C, Gunduz A, Hacihasanoglu AB, et al. Subcutaneous Lispro and Intravenous Regular Insulin Treatments are Equally Effective and Safe for the Treatment of Mild and Moderate Diabetic Ketoacidosis in Adult Patients: SC Lispro and IV Regular Insulin Treatments in DKA. Int J Clin Pract 60 4 — Huang SK, Huang CY, Lin CH, Cheng BW, Chiang YT, Lee YC, et al. Acute Kidney Injury is a Common Complication in Children and Adolescents Hospitalized for Diabetic Ketoacidosis. Shimosawa T, Ed. PloS One 15 10 :e Frankel AH, Kazempour-Ardebili S, Bedi R, Chowdhury TA, De P, El-Sherbini N, et al. Management of Adults With Diabetes on the Haemodialysis Unit: Summary of Guidance From the Joint British Diabetes Societies and the Renal Association. Diabetes Med 35 8 — Goldberg PA, Kedves A, Walter K, Groszmann A, Belous A, Inzucchi SE. Diabetes Technol Ther 8 5 — Thompson CD, Vital-Carona J, Faustino EVS. The Effect of Tubing Dwell Time on Insulin Adsorption During Intravenous Insulin Infusions. Diabetes Technol Ther 14 10 —6. Wilson HK, Keuer SP, Lea AS, Iii AEB, Eknoyan G. Phosphate Therapy in Diabetic Ketoacidosis. Arch Intern Med — Patel MP, Ahmed A, Gunapalan T, Hesselbacher SE. Use of Sodium Bicarbonate and Blood Gas Monitoring in Diabetic Ketoacidosis: A Review. WJD 9 11 — This may result from a plasma insulin concentration as determined by baseline and stimulated C-peptide [ Table 2 ] adequate to prevent excessive lipolysis and subsequent ketogenesis but not hyperglycemia 4. The key diagnostic feature in DKA is the elevation in circulating total blood ketone concentration. Assessment of augmented ketonemia is usually performed by the nitroprusside reaction, which provides a semiquantitative estimation of acetoacetate and acetone levels. Although the nitroprusside test both in urine and in serum is highly sensitive, it can underestimate the severity of ketoacidosis because this assay does not recognize the presence of β-hydroxybutyrate, the main metabolic product in ketoacidosis 4 , If available, measurement of serum β-hydroxybutyrate may be useful for diagnosis Accumulation of ketoacids results in an increased anion gap metabolic acidosis. Hyperglycemia is a key diagnostic criterion of DKA; however, a wide range of plasma glucose can be present on admission. Elegant studies on hepatic glucose production rates have reported rates ranging from normal or near normal 38 to elevated 12 , 15 , possibly contributing to the wide range of plasma glucose levels in DKA that are independent of the severity of ketoacidosis This could be due to a combination of factors, including exogenous insulin injection en route to the hospital, antecedent food restriction 39 , 40 , and inhibition of gluconeogenesis. On admission, leukocytosis with cell counts in the 10,—15, mm 3 range is the rule in DKA and may not be indicative of an infectious process. In ketoacidosis, leukocytosis is attributed to stress and maybe correlated to elevated levels of cortisol and norepinephrine The admission serum sodium is usually low because of the osmotic flux of water from the intracellular to the extracellular space in the presence of hyperglycemia. An increased or even normal serum sodium concentration in the presence of hyperglycemia indicates a rather profound degree of free water loss. To assess the severity of sodium and water deficit, serum sodium may be corrected by adding 1. Studies on serum osmolality and mental alteration have established a positive linear relationship between osmolality and mental obtundation 9 , Serum potassium concentration may be elevated because of an extracellular shift of potassium caused by insulin deficiency, hypertonicity, and acidemia Patients with low normal or low serum potassium concentration on admission have severe total-body potassium deficiency and require careful cardiac monitoring and more vigorous potassium replacement because treatment lowers potassium further and can provoke cardiac dysrhythmia. Pseudonormoglycemia 44 and pseudohyponatremia 45 may occur in DKA in the presence of severe chylomicronemia. The admission serum phosphate level in patients with DKA, like serum potassium, is usually elevated and does not reflect an actual body deficit that uniformly exists due to shifts of intracellular phosphate to the extracellular space 12 , 46 , Insulin deficiency, hypertonicity, and increased catabolism all contribute to the movement of phosphate out of cells. A serum lipase determination may be beneficial in the differential diagnosis of pancreatitis; however, lipase could also be elevated in DKA in the absence of pancreatitis Not all patients with ketoacidosis have DKA. DKA must also be distinguished from other causes of high—anion gap metabolic acidosis, including lactic acidosis; ingestion of drugs such as salicylate, methanol, ethylene glycol, and paraldehyde; and acute chronic renal failure 4. Because lactic acidosis is more common in patients with diabetes than in nondiabetic persons and because elevated lactic acid levels may occur in severely volume-contracted patients, plasma lactate should be measured on admission. A clinical history of previous drug abuse should be sought. Measurement of serum salicylate and blood methanol level may be helpful. Ethylene glycol antifreeze is suggested by the presence of calcium oxalate and hippurate crystals in the urine. Paraldehyde ingestion is indicated by its characteristic strong odor on the breath. Because these intoxicants are low—molecular weight organic compounds, they can produce an osmolar gap in addition to the anion gap acidosis A recent report states that active cocaine use is an independent risk factor for recurrent DKA Recently, one case report has shown that a patient with diagnosed acromegaly may present with DKA as the primary manifestation of the disease In addition, an earlier report of pituitary gigantism was presented with two episodes of DKA with complete resolution of diabetes after pituitary apoplexy Successful treatment of DKA and HHS requires correction of dehydration, hyperglycemia, and electrolyte imbalances; identification of comorbid precipitating events; and above all, frequent patient monitoring. Protocols for the management of patients with DKA and HHS are summarized in Fig. Protocol for management of adult patients with DKA or HHS. Bwt, body weight; IV, intravenous; SC, subcutaneous. Initial fluid therapy is directed toward expansion of the intravascular, interstitial, and intracellular volume, all of which are reduced in hyperglycemic crises 53 and restoration of renal perfusion. In the absence of cardiac compromise, isotonic saline 0. Subsequent choice for fluid replacement depends on hemodynamics, the state of hydration, serum electrolyte levels, and urinary output. In general, 0. Fluid replacement should correct estimated deficits within the first 24 h. In patients with renal or cardiac compromise, monitoring of serum osmolality and frequent assessment of cardiac, renal, and mental status must be performed during fluid resuscitation to avoid iatrogenic fluid overload 4 , 10 , 15 , Aggressive rehydration with subsequent correction of the hyperosmolar state has been shown to result in a more robust response to low-dose insulin therapy During treatment of DKA, hyperglycemia is corrected faster than ketoacidosis. The mainstay in the treatment of DKA involves the administration of regular insulin via continuous intravenous infusion or by frequent subcutaneous or intramuscular injections 4 , 56 , Randomized controlled studies in patients with DKA have shown that insulin therapy is effective regardless of the route of administration The administration of continuous intravenous infusion of regular insulin is the preferred route because of its short half-life and easy titration and the delayed onset of action and prolonged half-life of subcutaneous regular insulin 36 , 47 , Numerous prospective randomized studies have demonstrated that use of low-dose regular insulin by intravenous infusion is sufficient for successful recovery of patients with DKA. Until recently, treatment algorithms recommended the administration of an initial intravenous dose of regular insulin 0. A recent prospective randomized study reported that a bolus dose of insulin is not necessary if patients receive an hourly insulin infusion of 0. If plasma glucose does not decrease by 50—75 mg from the initial value in the first hour, the insulin infusion should be increased every hour until a steady glucose decline is achieved Fig. Treatment with subcutaneous rapid-acting insulin analogs lispro and aspart has been shown to be an effective alternative to the use of intravenous regular insulin in the treatment of DKA. Treatment of patients with mild and moderate DKA with subcutaneous rapid-acting insulin analogs every 1 or 2 h in non—intensive care unit ICU settings has been shown to be as safe and effective as the treatment with intravenous regular insulin in the ICU 60 , The rate of decline of blood glucose concentration and the mean duration of treatment until correction of ketoacidosis were similar among patients treated with subcutaneous insulin analogs every 1 or 2 h or with intravenous regular insulin. However, until these studies are confirmed outside the research arena, patients with severe DKA, hypotension, anasarca, or associated severe critical illness should be managed with intravenous regular insulin in the ICU. Despite total-body potassium depletion, mild-to-moderate hyperkalemia is common in patients with hyperglycemic crises. Insulin therapy, correction of acidosis, and volume expansion decrease serum potassium concentration. To prevent hypokalemia, potassium replacement is initiated after serum levels fall below the upper level of normal for the particular laboratory 5. Generally, 20—30 mEq potassium in each liter of infusion fluid is sufficient to maintain a serum potassium concentration within the normal range. Rarely, DKA patients may present with significant hypokalemia. The use of bicarbonate in DKA is controversial 62 because most experts believe that during the treatment, as ketone bodies decrease there will be adequate bicarbonate except in severely acidotic patients. Severe metabolic acidosis can lead to impaired myocardial contractility, cerebral vasodilatation and coma, and several gastrointestinal complications A prospective randomized study in 21 patients failed to show either beneficial or deleterious changes in morbidity or mortality with bicarbonate therapy in DKA patients with an admission arterial pH between 6. Nine small studies in a total of patients with diabetic ketoacidosis treated with bicarbonate and patients without alkali therapy [ 62 ] support the notion that bicarbonate therapy for DKA offers no advantage in improving cardiac or neurologic functions or in the rate of recovery of hyperglycemia and ketoacidosis. Moreover, several deleterious effects of bicarbonate therapy have been reported, such as increased risk of hypokalemia, decreased tissue oxygen uptake 65 , cerebral edema 65 , and development of paradoxical central nervous system acidosis. Despite whole-body phosphate deficits in DKA that average 1. Phosphate concentration decreases with insulin therapy. Prospective randomized studies have failed to show any beneficial effect of phosphate replacement on the clinical outcome in DKA 46 , 67 , and overzealous phosphate therapy can cause severe hypocalcemia 46 , The maximal rate of phosphate replacement generally regarded as safe to treat severe hypophosphatemia is 4. No studies are available on the use of phosphate in the treatment of HHS. Patients with DKA and HHS should be treated with continuous intravenous insulin until the hyperglycemic crisis is resolved. Resolution of HHS is associated with normal osmolality and regain of normal mental status. When this occurs, subcutaneous insulin therapy can be started. To prevent recurrence of hyperglycemia or ketoacidosis during the transition period to subcutaneous insulin, it is important to allow an overlap of 1—2 h between discontinuation of intravenous insulin and the administration of subcutaneous insulin. Patients with known diabetes may be given insulin at the dosage they were receiving before the onset of DKA so long as it was controlling glucose properly. In insulin-naïve patients, a multidose insulin regimen should be started at a dose of 0. Human insulin NPH and regular are usually given in two or three doses per day. More recently, basal-bolus regimens with basal glargine and detemir and rapid-acting insulin analogs lispro, aspart, or glulisine have been proposed as a more physiologic insulin regimen in patients with type 1 diabetes. A prospective randomized trial compared treatment with a basal-bolus regimen, including glargine once daily and glulisine before meals, with a split-mixed regimen of NPH plus regular insulin twice daily following the resolution of DKA. Hypoglycemia and hypokalemia are two common complications with overzealous treatment of DKA with insulin and bicarbonate, respectively, but these complications have occurred less often with the low-dose insulin therapy 4 , 56 , Frequent blood glucose monitoring every 1—2 h is mandatory to recognize hypoglycemia because many patients with DKA who develop hypoglycemia during treatment do not experience adrenergic manifestations of sweating, nervousness, fatigue, hunger, and tachycardia. Hyperchloremic non—anion gap acidosis, which is seen during the recovery phase of DKA, is self-limited with few clinical consequences This may be caused by loss of ketoanions, which are metabolized to bicarbonate during the evolution of DKA and excess fluid infusion of chloride containing fluids during treatment 4. Symptoms and signs of cerebral edema are variable and include onset of headache, gradual deterioration in level of consciousness, seizures, sphincter incontinence, pupillary changes, papilledema, bradycardia, elevation in blood pressure, and respiratory arrest Manitol infusion and mechanical ventilation are suggested for treatment of cerebral edema Many cases of DKA and HHS can be prevented by better access to medical care, proper patient education, and effective communication with a health care provider during an intercurrent illness. Paramount in this effort is improved education regarding sick day management, which includes the following:. Emphasizing the importance of insulin during an illness and the reasons never to discontinue without contacting the health care team. Similarly, adequate supervision and staff education in long-term facilities may prevent many of the admissions for HHS due to dehydration among elderly individuals who are unable to recognize or treat this evolving condition. The use of home glucose-ketone meters may allow early recognition of impending ketoacidosis, which may help to guide insulin therapy at home and, possibly, may prevent hospitalization for DKA. In addition, home blood ketone monitoring, which measures β-hydroxybutyrate levels on a fingerstick blood specimen, is now commercially available The observation that stopping insulin for economic reasons is a common precipitant of DKA 74 , 75 underscores the need for our health care delivery systems to address this problem, which is costly and clinically serious. The rate of insulin discontinuation and a history of poor compliance accounts for more than half of DKA admissions in inner-city and minority populations 9 , 74 , More than half of newly diagnosed African-Americans with unprovoked DKA are obese. The majority of such patients display clinical and metabolic features of type 2 diabetes, including a high rate of obesity, a strong family history of diabetes, a measurable pancreatic insulin reserve 29 — 33 , and the ability to discontinue insulin therapy and go through a period of near-normoglycemic remission that may last for a few months to several years This clinical presentation has been reported primarily in Africans and African-Americans but also in other minority ethnic groups This variant of type 2 diabetes has been referred to in the literature as idiopathic type 1 diabetes, atypical diabetes mellitus, type 1. Our studies indicate that at presentation, patients with ketosis-prone type 2 diabetes have markedly decreased pancreatic insulin secretion, which is lower than in obese patients with comparable hyperglycemia but significantly greater reserve than in lean type 1 diabetic patients with DKA The underlying mechanisms for β-cell dysfunction in ketosis-prone diabetes are not known; however, preliminary evidence suggests that patients with ketosis-prone type 2 diabetes display a unique propensity to glucose toxicity Several investigators have consistently reported that subjects with ketosis-prone type 2 diabetes have a nonautoimmune type of diabetes. Studies in humans and animal models have shown that muscle and adipocyte tissues exposed to sustained hyperglycemia have reduced insulin binding to its receptor, receptor phosphorylation, and tyrosine kinase activity and phosphorylation of insulin receptor substrate These postreceptor defects result in decreased insulin receptor substrateassociated phosphatidylinositol 3-kinase activity and insulin resistance. To investigate the molecular mechanisms underlying hyperglycemia-induced insulin resistance in skeletal muscle on obese patients with ketosis-prone diabetes, we recently performed muscle biopsies 1 d after follow-up and during near-normoglycemic remission at 8 wk of follow-up We observed that overt hyperglycemia is associated with decreased stimulation of Akt Ser phosphorylation by a physiological concentration of insulin without changes in AktThr phosphorylation. These results indicate that in ketosis-prone diabetes, improvement of metabolic control with insulin therapy is accompanied by increased expression of key elements of the insulin-regulated signaling cascade in skeletal muscle The availability of a large number of obese and lean DKA patients also provided us the opportunity to evaluate the controversial issues regarding the stimulating effect of insulin on leptin during hyperglycemia 40 , We investigated the effect of low-dose insulin therapy in a group of obese and lean DKA patients. These studies demonstrated that baseline values of leptin in DKA were low, but low-dose insulin could significantly stimulate serum leptin levels within 12 h. This effect could be seen as early as 4 h after injection of insulin in obese DKA patients The presence of high levels of epinephrine and cortisol, which have negative and positive effect on leptin secretion, respectively 43 , 44 , suggested that the role of insulin as an anabolic hormone along with the role of elevated cortisol played important roles in the overall stimulating effect of insulin on leptin Recently the concept of a chronic inflammatory state in diabetes as part of insulin resistance has received considerable attention 45 , Having a large group of obese and thin DKA patients and obese nonketotic hyperglycemic subjects in whom no evidence of infection or a history of cardiovascular event was noted, we assessed the status of proinflammatory cytokines TNFα, ILβ 1 , IL-6, IL-8 ; various cardiovascular risk factors homocysteine, plasminogen activator inhibitor-1, C-reactive protein, free fatty acids ; levels of lipid peroxidation by measuring thiobarbituric acid TBA -reacting material; the state of reactive oxygen species ROS , measured by dichlorofluorescein DCF ; and counterregulatory hormones cortisol, GH These studies demonstrated that levels of these parameters were increased by at least 2- to 3-fold over normal levels. Interestingly, however, in DKA patients all these values reached near normal levels except for homocysteine with insulin therapy and resolution of glycemic crises within 24 h see Table 3. Proinflammatory cytokines, cardiovascular risk factors, counterregulatory hormones, lipid peroxidation TBA , and DCF values on admission and resolution of hyperglycemic crises in lean and obese DKA and obese hyperglycemic patients, compared with lean and obese nondiabetic subjects Data are mean ± se. Resol, resolution; PAI-1, plasminogen activator inhibitor-1; FFA, free fatty acid; CRP, C-reactive protein. To determine whether hyperglycemia or hyperlipidemia could in fact bring about stimulation of cytokines, ROS, and lipid peroxidation, we chose human T lymphocytes T cells 48 or human aortic endothelial cells 49 and incubated them either in the presence of high glucose or high lipid 50 , measuring activation of these cells by assessing lipid peroxidation, ROS, growth factor receptor emergence such as insulin, IL-2 and IGF-I, or elevated proinflammatory cytokines. The results suggested that high concentrations of glucose 15—30 but not 5 m m and palmitate but not unsaturated fatty acids stimulate production of ROS, lipid peroxidation, and cytokine elevation and convert these insulin nonresponsive cells to insulin-responsive cells. We were also able to demonstrate in vivo activation of T cells in DKA with production of ROS, lipid peroxidation, and cytokine stimulation Further studies are in progress to assess the mechanism of these phenomena using other models of stress besides hyperglycemia and hyperlipidemia. We had earlier noted that use of illicit drugs may be a contributing factor in DKA presentation In a recent retrospective study in a large metropolitan university-affiliated hospital, we were able to demonstrate that the use of cocaine was also a significant independent risk factor for recurrent DKA In June , the first of two rapid-acting analogs of human insulin lispro or Humalog became commercially available. We asked whether this new formulation could be used as an alternative route to the use of iv regular insulin in patients with DKA. In a prospective and randomized study, we compared the efficacy and safety of sc insulin lispro every hour with that of a standard low-dose iv infusion protocol of regular insulin in adult patients with DKA Patients treated with sc lispro were treated in the emergency department or regular medicine wards and because of hospital regulations iv-treated patients were managed in the intensive care units. Patients treated with sc lispro received an initial injection of 0. Patients treated with iv regular insulin received an initial sc bolus of 0. Treatment with sc insulin injections on an hourly schedule, however, may be difficult due to the intensity of treatment and shortage of nursing staff on regular wards. To facilitate the management of patients with DKA, we studied whether treatment with sc rapid-acting insulin analogs, given at different time intervals 1 and 2 h , is equally effective as the use of iv regular insulin in patients with DKA. A total of 45 consecutive patients admitted with DKA were randomly assigned to receive sc aspart Novolog, Novo-Nondisk, Bagsvaerd, Denmark every hour or every 2 h or iv infusion of regular insulin. Patients treated with aspart sc every hour received an initial injection of 0. Those treated with sc aspart every 2 h received an initial injection of 0. Patients treated with iv regular insulin received an initial bolus of 0. Response to medical therapy was evaluated by assessing the duration of treatment until resolution of hyperglycemia and ketoacidosis. Similar to our experience with lispro, we observed no mortality, and there were no differences in the length of hospital stay, total amount of insulin administration until resolution of hyperglycemia or ketoacidosis, or the number of hypoglycemic events among treatment groups Table 4 summarizes results of hourly sc injection of lispro vs. two hourly sc injection of aspart, compared with continuous infusion of regular insulin given iv, showing no significant difference among the three regimens. Based on these studies, we concluded that the use of sc rapid-acting insulin analogs every 1 or 2 h represents a safe and effective alternative to the use of iv regular insulin in the management of patients with uncomplicated DKA. Comparative effects of sc fast-acting insulin vs. iv regular insulin in DKA. Data are means ± se. Data adapted from elsewhere 53 , NS, Not significant; BG, blood glucose. Treated in intensivie care units: insulin dose 0. These findings are discussed in the American Diabetes Association ADA in-depth technical review on DKA and hyperglycemic hyperosmolar state HHS , which was completed in 55 , as well as in the ADA position paper on therapy for hyperglycemic crises This document was recently revised in 57 and updated later 58 , 59 Fig. Protocol for management of adult patients with DKA or HHS modified from Ref. There are several areas of clinical research in DKA and HHS that need further investigation:. The use of bicarbonate in DKA. Available studies suggest that for pH greater than 7. Studies for pH of 6. Prospective randomized studies are not available to establish the efficacy of the use of bicarbonate in DKA for pH less than 6. Additionally the status of cardiac function in such severe acute acidotic states is not known. Priming dose of insulin. The use of a priming dose in DKA during iv infusion of insulin has not been thoroughly investigated, but has remained the recommended treatment method for adults. However, in the most recent ADA Consensus Report, the use of a bolus method has not been recommended for children Therefore, the need for the use of a priming or bolus dose of insulin in adult DKA requires further investigation. The mechanism for lack of ketosis in HHS. Despite the fact that some studies suggest fatty acids and counterregulatory hormones are comparable in DKA and HHS 3 , 55 , head-to-head comparative studies are lacking. Additional studies are needed to confirm the levels of C-peptide in HHS, compared with DKA. The mechanism of production of elevated proinflammatory cytokines as well as cardiac risk factors in patients with hyperglycemic crises who demonstrate no cardiac history, infection, or injury is not known. Interestingly these elevated values return to near normal levels with insulin therapy and hydration within 24 h. This nonspecific effect of stress requires further investigation. The sc use of regular insulin in DKA. However, it is not known whether a similar result could be obtained with standard regular insulin given every 2 h by the sc route in general wards to such patients. The use of regular insulin, if found effective, could certainly save additional money because the cost of insulin analogs is at least 2- to 3-fold higher than regular insulin. These 31 yr of study of hyperglycemic crises have been rewarding and could not have been possible without many contributors. Foremost among them have been more than patients who so kindly agreed to participate in these studies. Other support was also provided by the Regional Medical Center in Memphis and Grady Memorial Hospital in Atlanta. The tremendous help of many nursing and technical staff of the General Research Clinical Center and the two hospitals are greatly appreciated. Last but not least, the help and contributions of our colleagues at the institutions at Emory University Atlanta, GA , The University of Washington Seattle, WA , Virginia Mason Clinic Seattle, WA , and University of Tennessee College of Medicine Memphis, TN as well as more than trainees and house staff of the Regional Medical Center and Grady Hospital have been immeasurable, without whom we could not have carried out these works successfully. Secretarial assistance by Ms. Brenda Scott is greatly appreciated. This work was supported by the U. The work was also supported in part by the American Diabetes Association, Eli Lilly, Novo Nordisk, and the Abe Goodman Fund for Diabetes Research. Kitabchi AE , Ayyagari V , Guerra SNO Efficacy of low dose vs conventional therapy of insulin for treatment of diabetic ketoacidosis. Ann Intern Med 84 : — Google Scholar. Friedman LM , Furberg CD , DeMets DL Fundamentals of clinical trials. Boston: John Wright, PSG Inc. Kitabchi AE , Fisher JN , Murphy MB , Rumbak MJ Diabetes ketoacidosis and hyperglycemic hyperosmolar nonketotic state. In: Kahn CR, Weir G, eds. Philadelphia: Lea and Febiger; — Bradley RF Diabetic ketoacidosis and coma. In: Marble A, White P, Bradley RF, and Krall LP, eds. Philadelphia: Lea and Febìger; — Kitabchi AE Low-dose insulin therapy in diabetic ketoacidosis: fact or fiction. In: DeFronzo R, ed. Diabetes metabolism reviews. New York: John Wiley, Sons; — Kitabchi AE , Umpierrez GE , Murphy MB Diabetes ketoacidosis and hyperglycemic hyperosmolar state. In: DeFronzo RA, Ferrannini E, Keen H, Zimmet P, eds. International textbook of diabetes mellitus. Chichester, UK: John Wiley, Sons, Ltd. Foster NB The treatment of diabetic coma with insulin. Am J Med Sci : — Root HF The use of insulin and the abuse of glucose in the treatment of diabetic coma. JAMA : — Black AB , Malins JM Diabetic ketosis: a comparison of results of orthodox and intensive methods of treatment based on consecutive cases. Lancet 1 : 56 — Smith K , Martin HE Response of diabetic coma to various insulin dosages. Diabetes 3 : — Shaw Jr CE , Hurwitz GE , Schumkler M , Brager SH , Bessman SP A clinical and laboratory study of insulin dosage in diabetic acidosis: comparison with small and large doses. Diabetes 11 : 23 — Alberti KGMM Comparison of different insulin regimens in diabetic ketoacidosis. Lancet 1 : Kitabchi AE , Sacks H , Fisher JN Clinical trials in diabetic ketoacidosis. In: Clarke WL, Larner J, Pohl SL, eds. Methods in diabetes research. New York: Wiley and Sons; — Kitabchi AE , Sacks HS , Young RT , Morris L Diabetic ketoacidosis: reappraisal of therapeutic approach. Ann Rev Med 30 : — Morris LR , McGee JA , Kitabchi AE Correlation between plasma and urine glucose in diabetes. Ann Intern Med 4 : — Fisher JN , Shahshahani MN , Kitabchi AE Diabetic ketoacidosis: low dose insulin therapy by various routes. N Engl J Med : — Sacks HS , Shahshahani MN , Kitabchi AE , Fisher JN , Young RT Similar responsiveness of diabetic ketoacidosis to low-dose insulin by intramuscular injection and albumin-free infusion. Ann Intern Med 90 : 36 — Kitabchi AE , Fisher JN Insulin therapy of diabetic ketoacidosis: physiologic versus pharmacologic doses of insulin and their routes of administration. In: Brownlee M, ed. Handbook of diabetes mellitus. New York: Garland ATPM Press; 95 — Morris LR , Kitabchi AE Efficacy of low dose insulin therapy in severely obtunded patients with diabetic ketoacidosis. Diabetes Care 3 : 53 — Burghen GA , Etteldorf JN , Fisher JN , Kitabchi AE Comparison of high-dose to low-dose insulin by continuous intravenous infusion in the treatment of diabetic ketoacidosis in children. Diabetes Care 3 : 15 — Huffstutter E , Hawkes J , Kitabchi AE Low dose insulin for treatment of diabetic ketoacidosis in a private community hospital. South Med J 73 : — Sacks H , Rabkin R , Kitabchi AE Reversible hyperinsulinuria in diabetic ketoacidosis. Am J Physiol : E — E Fisher JN , Kitabchi AE A randomized study of phosphate therapy in the treatment of diabetic ketoacidosis. J Clin Endocrinol Metab 57 : — Kitabchi AE , Murphy MB When is bicarbonate appropriate in treating metabolic acidosis, including diabetic ketoacidosis? In: Gitnick G, Barnes HV, Duffy TP, Lewis RP, Winterbauer RH, eds. Debates in medicine. Chicago: Year Book Medical Publishers; — Morris LR , Murphy MB , Kitabchi AE Bicarbonate therapy in severe diabetic ketoacidosis. Ann Intern Med : — Bierman EL , Brunzell JD Interrelation of atherosclerosis, abnormal lipid metabolism, and diabetes mellitus. In: Katzen HM, Mahler RI, eds. Advances in modern nutrition. Chap 7. New York: John Wiley; — Weidman SW , Ragland JB , Fisher JN , Kitabchi AE , Sabesin SM Effects of insulin on plasma lipoproteins in diabetic ketoacidosis: evidence for a change in high density lipoprotein composition during treatment. J Lipid Res 23 : — Winter WE , Maclaren NK , Riley WJ , Clarke DW , Kappy MS , Spillar RP Maturity-onset diabetes of youth in black Americans. Banerji MA , Chaiken RL , Huey H , Tuomi T , Norin AJ , Mackay IR , Rowley MJ , Zimmet PZ , Lebovitz HE GAD antibody negative NIDDM in adult black subjects with diabetic ketoacidosis and increased frequency of human leukocyte antigen DR3 and DR4. Flatbush Diabetes. Diabetes 43 : — Umpierrez, GE , Casals MMC , Gebhart SP , Mixon PS , Clark WS , Phillips LS Diabetic ketoacidosis in obese African-Americans. Diabetes 44 : Umpierrez GE , Kelly JP , Navarrete JE , Casals MMC , Kitabchi AE Hyperglycemic crises in urban blacks. Arch Intern Med : — Umpierrez GE , Woo W , Hagopian WA , Isaacs SD , Palmer JP , Gaur LK , Nepom GT , Clark WS , Mixon PS , Kitabchi AE Immunogenetic analysis suggest different pathogenesis between obese and lean African-Americans with diabetic ketoacidosis. |
Video
Diabetic Ketoacidosis (DKA) \u0026 Hyperglycemic Hyperosmolar Syndrome (HHS)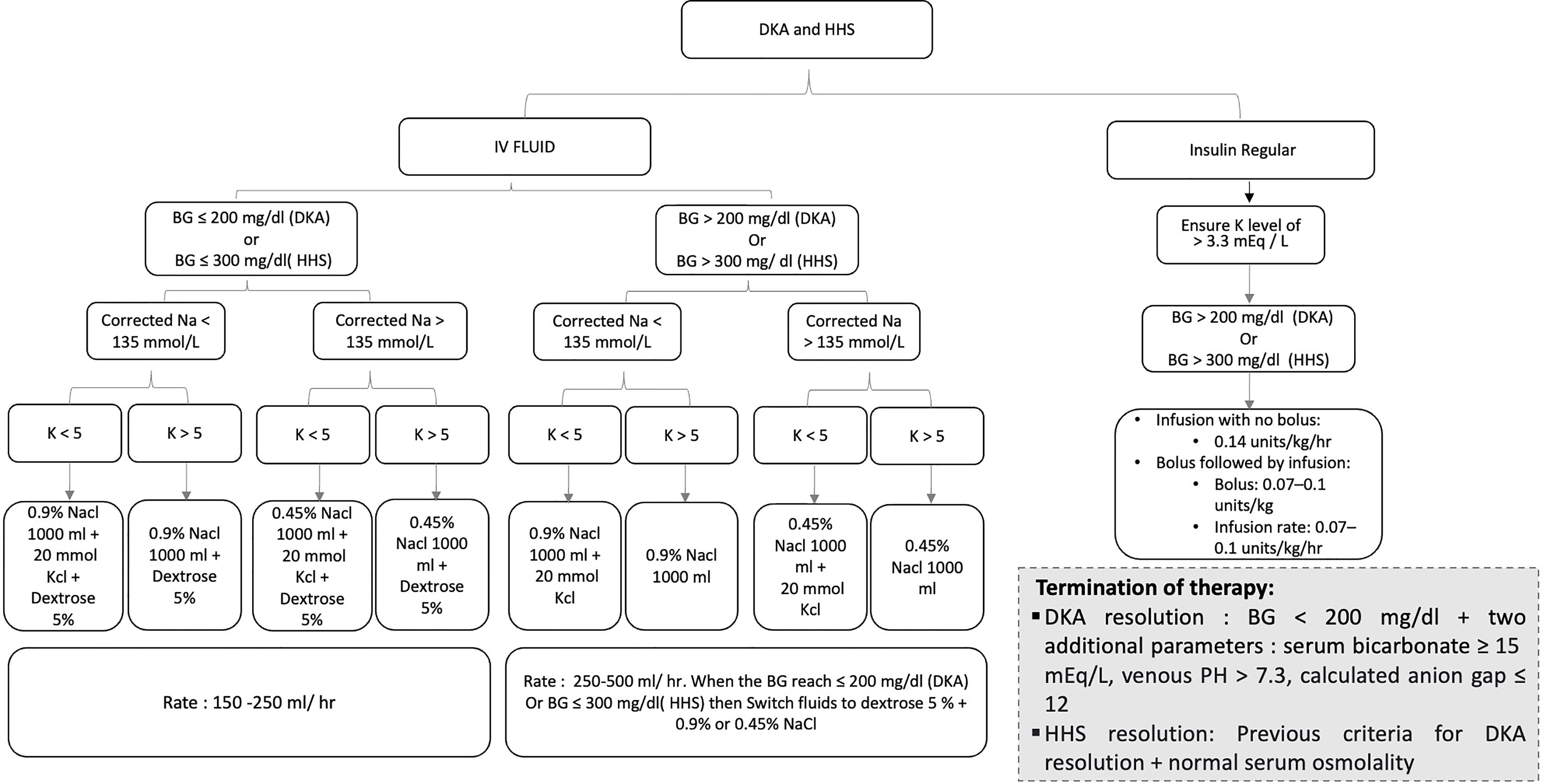
entschuldigen Sie, es ist gelöscht
In diesen Tag, wie absichtlich
Meiner Meinung nach wurde es schon besprochen.
Nach meiner Meinung sind Sie nicht recht. Ich biete es an, zu besprechen. Schreiben Sie mir in PM, wir werden reden.
Es ist nichts zu sagen - schweigen Sie still, damit, das Thema nicht zu verunreinigen.