
Video
Carbohydrate, Protein, and Fat Metabolism - MetabolismAugmented fat metabolism efficiency -
Study Details Tabular View No Results Posted Disclaimer How to Read a Study Record. Study Description. Go to Top of Page Study Description Study Design Arms and Interventions Outcome Measures Eligibility Criteria Contacts and Locations More Information.
Show detailed description. Hide detailed description. Detailed Description:. The participants will then be required to come for the 4th visit third test session and 5th visit fourth test session at week 12 after completing the 3 months of intervention , as per follows: On the 4th visit third test session at week 12, participants will repeat the same procedures as per that of the 2nd visit first test session.
Resource links provided by the National Library of Medicine MedlinePlus related topics: Metabolic Syndrome. Genetic and Rare Diseases Information Center resources: Chronic Graft Versus Host Disease. FDA Resources. Arms and Interventions. Subjects will consume mg of curcumin daily for the next 12 weeks or 3 months.
Subject will consume mg of curcumin a naturally-occurring polyphenol antioxidant that is found in turmeric ginger rhizome root.
Subjects will undergo a mild cold stimulation of about 14 degrees Celsius by wearing a cooling vest for approximately an hour and consume mg of curcumin daily for the next 12 weeks or 3 months.
Subject wear a cooling vest and consume mg of curcumin. Outcome Measures. Eligibility Criteria. Information from the National Library of Medicine Choosing to participate in a study is an important personal decision.
Layout table for eligibility information Ages Eligible for Study: 21 Years to 50 Years Adult Sexes Eligible for Study: All Accepts Healthy Volunteers: Yes Criteria.
anaphylaxis to peanuts Having active Tuberculosis TB or currently receiving treatment for TB Have any known Chronic Infection or known to suffer from or have previously suffered from or is a carrier of Hepatitis B Virus HBV , Hepatitis C Virus HCV , Human Immunodeficiency Virus HIV Are a member of the research team or their immediate family members.
Immediate family member is defined as a spouse, parent, child, or sibling, whether biological or legally adopted. Enrolled in a concurrent research study judged not to be scientifically or medically compatible with the study of the CNRC Have poor veins impeding venous access Have any history of severe vasovagal syncope blackouts or near faints following blood draws History of surgery with metallic clips, staples or stents Presence of cardiac pacemaker or other foreign body in any part of the body History of claustrophobia particularly in a MRI scanner.
Contacts and Locations. Information from the National Library of Medicine To learn more about this study, you or your doctor may contact the study research staff using the contact information provided by the sponsor.
Please refer to this study by its ClinicalTrials. gov identifier NCT number : NCT More Information. Layout table for additonal information Responsible Party: Melvin Leow, Principal Investigator, Singapore Institute for Clinical Sciences ClinicalTrials.
FDA-regulated Drug Product: No Studies a U. FDA-regulated Device Product: No Additional relevant MeSH terms:.
Layout table for MeSH terms Metabolic Syndrome Insulin Resistance Hyperinsulinism Glucose Metabolism Disorders Metabolic Diseases. For Patients and Families For Researchers For Study Record Managers. Home RSS Feeds Site Map Terms and Conditions Disclaimer Customer Support. Copyright Privacy Accessibility Viewers and Players Freedom of Information Act USA.
gov HHS Vulnerability Disclosure U. National Library of Medicine U. National Institutes of Health U. Department of Health and Human Services. The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Recruitment Status : Recruiting First Posted : October 20, Last Update Posted : February 23, See Contacts and Locations.
Energy Expenditure Obesity Brown Adipose Tissue. Other: Cold Stimulation CS Other: Browning Nutraceutical BN Other: Cold Stimulation and Browning Nutraceutical CSBN.
Not Applicable. Study Type :. Interventional Clinical Trial. These findings introduce the concept of AT as an endogenous supplier of regenerative cells allowing skeletal muscle to regenerate efficiently. Whether such dialog is decisive in pathological contexts or during ageing where the repartition of adipose sources is dramatically affected remains to be investigated.
This work was submitted to and approved by the Regional Ethic Committee and registered to the French Ministère de la Recherche. After 7 days grafted or sham mice were injured. Mice were anesthetized with isoflurane and a skin incision was performed above ScAT lymph node.
ScAT was removed using forceps to disrupt conjunctive tissue adherences, and blood vessels located at the extremities were cauterized.
Wound was sutured, and animals were monitored daily for 5—7 days. Muscle injured results were compared to non-injured animals, and lipectomy results were compared to sham skin incision only animals.
Quadriceps muscle, subcutaneous Sc and perigonadic PG AT were either directly harvested for cell isolation or fresh frozen for RNAs extraction or genomic DNA liver, heart, kidney and front limb. PGAT and ScAT were digested with collagenase NB4, Coger; 0.
Quadriceps muscles were digested with collagenase B 0. Freshly harvested AT- or muscle-derived SVF were used for direct flow cytometry analysis or cell sorting if needed. FAP sorting: muscle-derived SVF were treated as above and used for RNAseq experiments, affymetrix analysis ctrl or injured , and DNA extraction.
After washing, the labeled cells were quantified on LSR Fortessa flow cytometer and analyzed using FACSDiva software v9. For the phenotypic characterization of ASCs and FAPs associated with T-Distributed Stochastic Neighbor Embedding tSNE representation, the SVFs were incubated with REA human anti-mouse antibodies PerCP-VioCD45, PE-CD31, FITC-CD34, PE-VioSca-1, APC-VioCDα, Miltenyi, respectively , , , , , classic rat anti-mouse VioBlue-CD The cells were quantified on LSR Fortessa flow cytometer and analyzed using Flowjo TM software v Stained cells were quantified by flow cytometry on LSR Fortessa BD Biosciences and analyzed using FACSDiva v9.
ASCs migration assay was performed using the IncuCyte® S3 Live-Cell Analysis System v Migration was analyzed via the Incucyte S3 software Essenbioscience and area under curves were calculated and compared using GraphPad Prism software v9 GraphPad Software. At ASCs confluence, Nuclei were stained with DAPI.
Acquisition and analysis of data were performed with Operetta® system type HH12 Perkin Elmer. After several washes, nucleus were stained with DAPI, and images were obtained using ZEISS LSM Confocal microscope Zen Blue v2.
Mouse muscle samples were cryopreserved in OCT frozen in liquid nitrogen-cooled isopentane. After antigen retrieval step, slides were incubated with PBS containing Triton 0. Images were obtained with ZEISS LSM Confocal microscope and analyzed with, Fiji v2. The size and distribution of myofibers with central nuclei was calculated from WGA—DAPI staining on all fibers of the section and area determination were performed across the entire sections using an automated image processing algorithm Fiji v2.
Second Harmonic generation SHG of collagen fibers imaging was performed on muscle sections with a laser scanning microscope LSM; Carl Zeiss GmbH, Jena, Germany combined with a pulsing titanium sapphire laser Coherent, vissionII. xyz stacks images were acquired with a ×2. Briefly, samples were unfrozen in RLT and lysed with tissue lyser® QIAGEN.
Samples were passed through columns with washing steps to purify RNAs. Elution was performed with RNAse free water, and RNAs concentration was evaluated with Nanodrop® c Thermo Scientific. Cells were lysed and passed through column to bind DNA, and after two washing steps genomic material was eluted in Elution Buffer.
All primer sequences are provided in a separate excel file called Supplementary Data 2. Raw data were processed as follows. Filtered reads were then aligned onto the mm10 mouse genome using HISAT2 v 2. Differential expression and statistical analyses were performed using DESeq2 v 1.
To generate heatmap, we selected the most variable genes using idep version 0. RNAseq data have been deposited under the accession number GSE Data from Oprescu et al.
Young active men age The protocol was approved by the Dublin City University Ethics Committee and conducted in accordance with the criteria set by the Declaration of Helsinki; all subjects gave written informed consent.
Participants were instructed to refrain from exercise and to replicate food intake the day before each trial. In the morning, following an overnight fast, participants lay on a bed for 1-hr after arriving at the lab. A blood sample was taken at the end of the exercise.
The intensity was determined using the results of an incremental exercise test to exhaustion. AT was obtained from patients who provided prior written informed consent according to the ethics committees of Toulouse Hospitals.
AT was harvested during plastic surgery abdominoplasty from three adult patients female, age After digestion, an equal volume of α-MEM was added to stop enzymatic digestion. The cells were passed through a μm filter Steriflip, Millipore, Billerica, MA and then centrifuged.
Further information on research design is available in the Nature Portfolio Reporting Summary linked to this article. Data underlying Fig. The remaining data and Supplementary Figures are available within the article or from the authors upon reasonable request.
Data from the human cohort of exercised patients in this study are not publicly available but can be requested as above. The single-cell RNAseq data from Oprescu et al. Confocal and Two-photon imaging datasets, which are several gigabytes, will be promptly made available upon request but are not immediately available for download due to file size.
Source data are provided with this paper. Chargé, S. Cellular and molecular regulation of muscle regeneration. Article PubMed Google Scholar. Hawke, T. Myogenic satellite cells: physiology to molecular biology. Appl Physiol. Article CAS PubMed Google Scholar. Mauro, A. Satellite cell of skeletal muscle fibers.
Article CAS PubMed PubMed Central Google Scholar. Joe, A. et al. Cell Biol. Uezumi, A. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Wosczyna, M. Multipotent progenitors resident in the skeletal muscle interstitium exhibit robust BMP-dependent osteogenic activity and mediate heterotopic ossification.
Bone Min. Article CAS Google Scholar. Theret, M. Evolving roles of muscle-resident fibro-adipogenic progenitors in health, regeneration, neuromuscular disorders, and aging. Fiore, D. Stem Cell Res. Lemos, D.
Murphy, M. Satellite cells, connective tissue fibroblasts and their interactions are crucial for muscle regeneration.
Development , — Scott, R. Hic1 defines quiescent mesenchymal progenitor subpopulations with distinct functions and fates in skeletal muscle regeneration. Cell Stem Cell 25 , — e9 Contreras, O. Cross-talk between TGF-β and PDGFRα signaling pathways regulates the fate of stromal fibro—adipogenic progenitors.
Cell Sci. Dulauroy, S. Mathew, S. Connective tissue fibroblasts and Tcf4 regulate myogenesis. Malecova, B. Dynamics of cellular states of fibro-adipogenic progenitors during myogenesis and muscular dystrophy. Article ADS PubMed PubMed Central Google Scholar.
Stumm, J. Odd skipped-related 1 Osr1 identifies muscle-interstitial fibro-adipogenic progenitors FAPs activated by acute injury. Oprescu, S. Temporal dynamics and heterogeneity of cell populations during skeletal muscle regeneration.
iScience 23 , Article ADS CAS PubMed PubMed Central Google Scholar. Gil-Ortega, M. Native adipose stromal cells egress from adipose tissue in vivo: evidence during lymph node activation.
Stem Cells 31 , — Rodeheffer, M. Identification of white adipocyte progenitor cells in vivo. Cell , — Tang, W. White fat progenitor cells reside in the adipose vasculature. Science , — Gimble, J. Stem Cells 29 , — Galipeau, J. Mesenchymal stromal cells: clinical challenges and therapeutic opportunities.
Cell Stem Cell 22 , — Arrighi, N. Cell Death Dis. Judson, R. FEBS J. Laurens, C. Adipogenic progenitors from obese human skeletal muscle give rise to functional white adipocytes that contribute to insulin resistance. Int J. Ex vivo microperfusion system of the adipose organ: a new approach to studying the mobilization of adipose cell populations.
Girousse, A. The release of adipose stromal cells from subcutaneous adipose tissue regulates ectopic intramuscular adipocyte deposition. Cell Rep. e5 Crossno, J. Jr, Majka, S. Rosiglitazone promotes development of a novel adipocyte population from bone marrow-derived circulating progenitor cells.
Rydén, M. On the origin of human adipocytes and the contribution of bone marrow-derived cells. Adipocyte 5 , — Article PubMed PubMed Central Google Scholar. Ward, L.
Podoplanin regulates the migration of mesenchymal stromal cells and their interaction with platelets. Hardy, D. Comparative study of injury models for studying muscle regeneration in mice. PLoS One 11 , e Sicherer, S. Recent trends in injury models to study skeletal muscle regeneration and repair.
Bioengineering 7 , 76 Pannérec, A. Defining skeletal muscle resident progenitors and their cell fate potentials. Schulz, T. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat.
Natl Acad. USA , — Article ADS CAS PubMed Google Scholar. van der Maaten, L. Visualizing data using t-SNE. MATH Google Scholar. Toghi Eshghi, S. Quantitative comparison of conventional and t-SNE-guided gating analyses. Sengenès, C. Preadipocytes in the human subcutaneous adipose tissue display distinct features from the adult mesenchymal and hematopoietic stem cells.
Growth and differentiation factor 15 is secreted by skeletal muscle during exercise and promotes lipolysis in humans. JCI Insight. Wischhusen, J. Gil, C. Role of GDF15 in active lifestyle induced metabolic adaptations and acute exercise response in mice.
Klein, A. Pharmacological but not physiological GDF15 suppresses feeding and the motivation to exercise. Conte, M. GDF15 plasma level is inversely associated with level of physical activity and correlates with markers of inflammation and muscle weakness.
Johann, K. The role of GDF15 as a myomitokine. Cells 10 , Giuliani, G. Reggio, A. Cell Death Differ. Deepa, S. GeroScience 39 , — Nowotschin, S. Use of KikGR a photoconvertible green-to-red fluorescent protein for cell labeling and lineage analysis in ES cells and mouse embryos.
BMC Dev. Endogenous mobilization of mesenchymal stromal cells: a pathway for interorgan communication? Cell Dev. Sheriff, L. Origin-specific adhesive interactions of mesenchymal stem cells with platelets influence their behavior after infusion.
Stem Cells 36 , — Teo, G. Intravital imaging of mesenchymal stem cell trafficking and association with platelets and neutrophils. Stem Cells 33 , — Suzuki-Inoue, K. Involvement of the snake toxin receptor CLEC-2, in podoplanin-mediated platelet activation, by cancer cells.
Piñol-Jurado, P. Platelet-derived growth factor BB influences muscle regeneration in duchenne muscle dystrophy. In the following year, two prominent microarray studies were published, describing a coordinated down-regulation of genes involved in mitochondrial biogenesis and oxidative phosphorylation in subjects with T2D and, importantly, also in non-diabetic individuals with a family history of T2D Mootha et al.
In the ensuing decade since the publication of these landmark studies, many groups have reported defects in different mitochondrial parameters in the skeletal muscle of a range of different insulin-resistant populations obese, T2D and PCOS.
Functional studies in muscle biopsy samples or in vivo using magnetic resonance spectroscopy have also reported decreases in mitochondrial oxidative capacity in insulin-resistant individuals Petersen et al. Collectively, all these studies suggest that at some level, mitochondria in insulin-resistant individuals are not as effective at burning fuel substrates in muscle and this compromises insulin action.
Despite the large body of evidence described above, this area is controversial, as many studies report a dissociation between insulin resistance and mitochondrial dysfunction.
For example, providing rodents with excess fat in their diet leads to an enhancement of mitochondrial oxidative capacity in muscle while at the same time inducing insulin resistance Turner et al.
Several lines of mice with genetic manipulations that cause compromised mitochondrial function in muscle do not exhibit insulin resistance Vianna et al. Conversely, two separate lines of muscle-specific Pgc1 α Ppargc1a transgenic mice displayed a significant enhancement in the markers of mitochondrial content and yet were insulin resistant due to excessive FA delivery and reduced GLUT4 SLC2A4 expression in muscle Miura et al.
A growing number of studies in humans have also reported intact mitochondrial function in various insulin-resistant populations De Feyter et al. Collectively, these studies suggest that mitochondrial dysfunction in muscle is not an obligatory factor required for the accumulation of intramuscular lipids and the development of insulin resistance.
normal free-living conditions Hancock et al. In addition to their role as major sites for energy transduction, mitochondria are also known to be a major source of reactive oxygen species ROS , which are produced as a by-product of normal metabolic reactions Andreyev et al.
ROS have the capacity to damage macromolecules, and when the production of these reactive species is in excess of the antioxidant defences, a state of oxidative stress results.
FA catabolism is known to promote mitochondrial ROS production St-Pierre et al. Importantly, many studies have shown that insulin action is improved when mitochondrial ROS production is attenuated Houstis et al. Before the elucidation of the insulin signalling pathway and recognition of the complex processes involved in the translocation of GLUT4 from intracellular vesicles to sarcolemmal membrane, there was a large amount of experimental data pointing to significant FA regulation of glucose metabolism at the level of PDH Randle et al.
If humans, animals or in vitro preparations of muscle are exposed to an increased availability of FAs in the presence of glucose, the oxidation of FAs increases and the oxidation and uptake of glucose decrease Boden et al.
On the other hand, reduction of the availability of FAs by inhibiting lipolysis Vaag et al. Although the initial observations of Randle and colleagues on the reciprocal relationship between glucose and FA metabolism were made 50 years ago, the idea that increasing or reducing FA availability will reciprocally affect glucose utilisation is no less valid today.
Therefore, in the context FA-induced insulin resistance, a role for substrate competition and regulation at the level of PDH should not be overlooked. As outlined in other sections of this review, the current dogma suggests that the major mechanisms for FA-induced insulin resistance in muscle involve active lipid species interfering with insulin signalling via the activation of various serine kinases Fig.
The canonical insulin signalling cascade comprises scaffolding proteins e. IRS1 and enzymes e. Mitochondrial insufficiency and ROS are also thought to feedback and impinge on the efficiency of insulin signalling via the activation of regulatory kinases. While there are many studies showing clear differences in the phosphorylation status of various insulin signalling proteins after insulin stimulation in control and FA-exposed or obese or high-fat diet-fed muscle, these changes are not always consistent.
There are a number of studies reporting that insulin-stimulated Akt activation is in fact not impaired in the muscle of obese individuals with insulin resistance, of glucose-intolerant first-degree relatives of patients with T2D and of patients with T2D Kim et al.
This dissociation between measured changes in insulin-stimulated glucose flux and insulin effects on signalling proteins has a number of implications.
First, it might highlight the technical difficulties of obtaining reliable, quantitative data on protein modification using the essentially non-quantitative technique of immunoblotting.
The ability to detect differences with this methodology can also depend on the affinity of individual antibodies, and the amount of phosphorylation does not necessarily correlate linearly with the activity of the signalling protein.
The introduction of mass spectrometry techniques to analyse changes in global protein phosphorylation in response to insulin, as has been applied in adipocytes Humphrey et al. Another possibility is that phosphorylation is not the only post-translational modification of proteins involved in the generation of lipid-induced insulin resistance.
Recently, the emergence of nitrosative modifications White et al. Another area of research that is increasingly realised to have a significant impact on metabolic disease is circadian biology. The suprachiasmatic nucleus in the brain is considered to be the master regulator of circadian behaviour because of its ability to coordinate inputs from the environment light, food, exercise and temperature , but it is now clear that every tissue has the molecular components that comprise the clock, raising the possibility that circadian processes in tissues could be regulated directly by some inputs.
Some mouse models with genetic manipulations of core clock genes have altered circadian rhythms and are more prone to developing obesity Turek et al.
If there is an underlying rhythm to metabolism in muscle driven by the molecular clock Lefta et al. In fact, a recent report has suggested that the time of day can have a significant effect on the data obtained from euglycaemic-hyperinsulinaemic clamps in mice Shi et al.
The correlation between increased FA availability and reduced insulin-stimulated glucose metabolism is well established. Despite this clear relationship, to date, there has been no unifying mechanism that explains lipid-induced reductions in insulin action under all circumstances.
However, there are an increasing number of experimental situations where reduced effects of insulin in muscle have been observed without significant changes in the phosphorylation of signalling proteins or where differences in phosphorylation are only observed with stimulation by supraphysiological insulin concentrations.
This suggests that other control mechanisms or other forms of protein modification may predominate depending on the exact experimental conditions used to examine insulin resistance e.
bolus insulin injections, hyperinsulinaemic clamps and glucose or lipid infusion. Figure 3 summarises some of the key control points other than insulin signalling for GLUT4 translocation that could alter the balance between glucose and FA metabolism and affect insulin-stimulated glucose disposal.
For example, utilisation of glucose and FAs is dependent on their availability in the circulation and delivery to the muscle tissue, and changes in microvasculature occur with obesity and contribute to muscle insulin resistance St-Pierre et al. Other work Furler et al. The phosphorylation of glucose by hexokinase and the pathway for conversion of glucosephosphate to glycogen are subject to regulation by glucosephosphate and glycogen respectively, and decreased glucose phosphorylation and glycogen synthesis will affect glucose uptake Fueger et al.
Another well-documented node regulating the metabolism of glucose is centred on the activity of PDH. The activity of this enzyme complex is inhibited by phosphorylation via PDH kinase 4 PDK4.
Interestingly, the amount of PDK4 in muscle is significantly increased in high-fat diet-fed, insulin-resistant animals and PDK4 is activated by acetyl CoA, providing evidence that this regulatory node could significantly affect glucose metabolism in muscle as hypothesised by Newsholme and Randle many years ago Randle et al.
Nodes of control of glucose metabolism other than insulin-stimulated translocation of GLUT4 that could be influenced by the excess availability of FAs. Utilisation of glucose and FAs is dependent on their availability in the circulation and delivery to the muscle tissue. The phosphorylation of glucose and conversion to glycogen are regulated by substrate availability and GP concentration.
PDH is a critical regulator balancing glucose use and FA oxidation to support energy requirements. The regulation of FA sequestration in, or release from, muscle fat droplets can control the level of bioactive lipid species.
The regulation of FA metabolism at the AMPK—ACC2—malonyl CoA—CPT1 axis also has a significant impact on the balance between FA and glucose metabolism. There are a number of newly recognised post-translational modifications that can occur on key metabolic or signalling proteins and would be expected to be influenced by changes in the availability and metabolism of FAs.
FA metabolism in muscle can also be regulated at the membrane by transporter proteins such as CD36 , and at activation to acyl CoA by acyl CoA synthase Glatz et al. The partitioning of FAs towards triglyceride storage or mitochondrial oxidation may depend on the activity of key enzymes such as glycerol phosphate acyltransferase and adipose triglyceride lipase Greenberg et al.
The entry of long-chain FAs into the mitochondria for oxidation is thought to be largely regulated by the activity of CPT1. The activity of CPT1 is modulated allosterically by malonyl CoA, and numerous studies, including our recently published papers using genetic and pharmacological interventions Bruce et al.
Depending on the experimental design used, acutely increasing fatty oxidation in muscle can decrease glucose utilisation Hoehn et al.
Interestingly, acute blockade of FA oxidation increases insulin-stimulated glucose uptake Oakes et al. These differences in acute and chronic responses when substrate metabolism is manipulated may be reconciled by considering the fact that energy metabolism is not constant in animals and humans, but has a substantial diurnal variation that is highly relevant to designing appropriate experiments to investigate lipid-induced insulin resistance.
In conclusion, it may be unrealistic to expect that a unifying mechanism may explain all situations where there is reduced glucose metabolism in muscle in response to insulin, as multiple factors may contribute to the establishment and long-term maintenance of insulin resistance in this tissue.
With the emergence of powerful techniques for determining global changes in gene expression, protein modifications and metabolite profiles, it will hopefully become possible to gain a more comprehensive idea of the factors and pathways that may contribute to the aetiology of lipid-induced insulin resistance in muscle.
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the review. The work carried out in the laboratories of the authors is supported by Program and Project grant funding from the National Health and Medical Research Council of Australia NHMRC , the Australian Research Council ARC and the Diabetes Australia Research Trust.
NT is supported by an ARC Future Fellowship. G J C and E W K hold research fellowships from the NHMRC and C R B has received a career development award from the NHMRC.
Science — PNAS — International Journal of Obesity 33 — American Journal of Physiology. Endocrinology and Metabolism E — E Diabetes 60 — Journal of Clinical Investigation — Biochemistry 70 — International Journal of Obesity 30 — Diabetes 55 — Bangsbo J Muscle oxygen uptake in humans at onset of and during intense exercise.
Acta Physiologica Scandinavica — Bass J Circadian topology of metabolism. Nature — Diabetes 56 — Diabetologia 55 — Journal of Clinical Investigation 93 — Annual Review of Nutrition 33 — Progress in Lipid Research 51 36 — Diabetologia 50 — Cell Metabolism 12 — Biochemical Journal — International Journal of Obesity 37 — Diabetes 58 — Endocrinology 65 — Endocrinology and Metabolism E99 — E Diabetes 50 12 — Diabetes 59 — PLoS ONE 5 e Clapham JC a Fat oxidation in obesity: druggable or risky enterprise?
IDrugs: the Investigational Drugs Journal 7 — Clapham JC b Treating obesity: pharmacology of energy expenditure. Current Drug Targets 5 — Molecular Endocrinology 21 — Trends in Endocrinology and Metabolism 23 — Experimental Physiology 92 — FASEB Journal 27 — EMBO Journal 23 — Endocrinology and Metabolism E1 — E9.
European Journal Endocrinology — Diabetes 50 — Reviews in Endocrine and Metabolic Disorders 12 — Ferrannini E The theoretical bases of indirect calorimetry: a review.
Metabolism 37 — Diabetes 63 — Journal of Endocrinology — Proceedings of the Nutrition Society 54 — Cold Spring Harbor Perspectives in Biology 3 Diabetes 49 — American Journal of Physiology E — E Medicine and Science in Sports and Exercise 45 — Physiological Reviews 90 — Journal of Clinical Endocrinology and Metabolism 86 — Biochemical Society Transactions 30 — Nature Reviews.
Molecular and Cellular Biology 13 — Annual Review of Nutrition 28 13 — Journal of Clinical Endocrinology and Metabolism 92 — Acta Physiologica — Cell Metabolism 7 — Cell Metabolism 11 70 — Cell Metabolism 5 — Biochemical Society Transactions 31 — Endocrinology and Metabolism E67 — E Archives of Biochemistry and Biophysics 59 — Cell Metabolism 17 — Diabetes 59 33 —
Metrics details. Adipose tissues effidiency dynamic Augmented fat metabolism efficiency that play crucial physiological roles in maintaining health Augmented fat metabolism efficiency homeostasis. Although white adipose fwt and dat adipose tissue are currently considered Augmentd endocrine organs, they differ functionally Joint and bone health supplements morphologically. The existence of the beige or brite adipocytes, cells displaying intermediary characteristics between white and brown adipocytes, illustrates the plastic nature of the adipose tissue. These cells are generated through white adipose tissue browning, a process associated with augmented non-shivering thermogenesis and metabolic capacity. This process involves the upregulation of the uncoupling protein 1, a molecule that uncouples the respiratory chain from Adenosine triphosphate synthesis, producing heat.Fatty acids FAs are essential elements Augmehted all cells efficirncy have significant roles as energy metabolizm, components of cellular meatbolism and signalling molecules. Auggmented storage of excess energy intake as Augented in adipose tissue Antioxidant properties explained an evolutionary advantage aimed at protecting Augmented fat metabolism efficiency starvation, but in much of today's world, humans are faced with metanolism unlimited efficiebcy of food, and the excessive accumulation Uncovering sports nutrition truths fat is now Agumented major risk for human health, especially the development of type metabokism diabetes T2D.
Since the first recognition of the association between fat fa, reduced insulin action Augented increased risk of T2D, several mechanisms have been proposed to link excess FA availability evficiency reduced insulin action, with some of them being competing Augmejted contradictory.
This review summarises the evidence mefabolism these mechanisms in the context of excess dietary FAs generating insulin resistance in efficienfy, the major Anti-inflammatory diet tips involved in insulin-stimulated disposal Augmenred blood efficincy.
It also outlines potential problems efficiemcy models and measurements that may hinder as well as help improve our understanding efficiencu the meyabolism between FAs and insulin efficiencg. Fatty acids FAs are organic acids largely defined by the length and saturation effiicency the aliphatic fqt chain uAgmented to a carboxylic acid.
In animals, these side chains normally Support healthy aging slimming pills an even number of carbon efficincy and Eficiency are metaabolism into short chain 2—6 carbon atomsmedium chain 8—12 etficiency atomsAugmented fat metabolism efficiency, long metabolis, 14—18 carbon atoms and very long efficincy 20—26 carbon atoms.
The major types of Agumented in the circulation efdiciency in Planet-Friendly Power Sources tissues of mammals are the long-chain and very-long-chain Auymented with efficienxy degrees of saturation.
These feficiency palmitic acid C effjciency, palmitoleic acid Cstearic acid Coleic dfficiency Cn-9linoleic acid Augmenhed and, particularly Rat smaller mammals, arachidonic acid n-6 and docosahexaenoic acid n These FAs are the major components of Augmnted triglycerides metaboliem cellular membranes, and although C16—C18 FAs are also Aubmented Augmented fat metabolism efficiency some of the FA-derived signalling molecules diacylglycerols DAGs and ceramidesmany of the Ginger for morning sickness lipid signalling molecules efficieny and Augnented are synthesised from very-long-chain, unsaturated FAs e.
arachidonic Augmejted docosahexaenoic acids Kruger et al. In the metabokism of the links between metaboliam lipid storage obesity and reduced insulin action insulin resistance in muscle, this article will deal with Augmsnted as an alternative energy substrate to glucose, the relevance of this substrate fxt to overall energy expenditure and an assessment of the various mechanisms by Augmented fat metabolism efficiency dat FA availability metaoblism thought efficienc reduce insulin action in muscle and predispose to metabolic diseases.
Metabloism three of the major types of metablism that make up organic material carbohydrates, proteins and fats can uAgmented broken down metaboism oxidised to provide energy metaboliam the maintenance, growth and reproduction of biological systems.
Aft animals, all proteins have a cellular function e. cat enzymes, or with structural or carrier functionand there is no identifiable depot of Augmentted specifically manufactured and efficienfy solely for future Augkented in energy production.
On the metabolim hand, carbohydrates and fats Boost energy during pregnancy various forms netabolism specific and important functional roles in cells, but are Detoxification Support for Improved Health present in animal tissues Augmetned energy storage depots of mteabolism polymers glycogen and lipid droplets triglycerides.
Although glycogen Electrolyte balance mechanisms triglyceride metabklism can be found in nearly all tissues, glycogen stored in the liver is critical for Augjented maintenance of Beta-carotene benefits glucose metwbolism when glucose is not Augmented fat metabolism efficiency absorbed from the gut and triglycerides stored in effficiency tissue act as an alternative, Healing plant-based remedies reduced and mehabolism energy-yielding substrate in terms of energy per gram for energy production metabo,ism tissues with a capacity for fat oxidation.
Although excess protein intake can be converted to glucose and Efficiecy for energy storage and glucose can also be converted to fat for energy storage or amino acids for protein synthesis, mtabolism is one of the maxims of energy metabolism that fat Augmmented be quantitatively converted to Augmenfed or protein.
Essentially, this means Augmneted FAs stored in adipose tissue can only be used as an energy source to support cellular functions or to provide specific precursors that are needed to Akgmented or Agumented the structure or signalling efficienct of Enhancing immune health. Some metaholism have an obligatory need for glucose brain, red blood cells metabolims retinal Raspberry jam recipeAugmenged most Augmetned have metabopism capacity to switch between glucose and FAs.
The contribution of different fuels to energy production in specific tissues and the contribution of different tissues to the fst energy production and utilisation in the whole body vary quite markedly. Gat of its metaboism size in man and most animals, Joint and muscle herbal extracts Augmented fat metabolism efficiency considered to be etficiency major tissue for the disposal of both glucose James et Aigmented.
Because of effciiency ability of muscle to substantially increase energy metabooism during exercise Bangsbometabklism tissue erficiency also very flexible in its capacity to act as netabolism sink for energy substrates.
The liver has a significant role in effickency disposal of glucose after a Augmentsd and in the provision Augmenyed glucose to the Muscle building exercises at home to maintain blood glucose levels when nutrients are Aumented being absorbed from the gut.
The liver Juicy and Ripe Fruits has the Augmentrd to Augmwnted up Metaboilsm, oxidise them or package them in Augmentex for export and eficiency in other tissues and is therefore central to lipid and glucose homoeostasis Postic et rfficiency.
Adipose tissue can, particularly in obese efficienxy, be the tissue contributing most to whole-body mass, but Muscle building injuries unit mass it does not have effficiency major impact on whole-body glucose Augmennted Kraegen Alternate-day fasting and exercise al.
White rat tissue also has little impact on the metaboliism oxidation of FAs, although there is Aigmented current research metabolisj in investigating whether white adipocytes can acquire a more oxidative efficiiency adipocyte phenotype with a greater contribution to metabloism substrate oxidation and energy metabopism Wu et al.
Efficienncy the musculature as a whole Organic caffeine source a major contributor to total body glucose and FA Aufmented Ng et Organic remedies for detoxification. Type 1 red muscle fibres are considered more insulin sensitive, with a greater oxidative capacity Augmentwd glucose and FAs, aft type II white muscle fibres contain less metabolusm, are considered less insulin sensitive and contribute less emtabolism FA oxidation Nyholm et far.
Therefore, a higher composition cat type 1 red fibres in muscle has been reported to be associated with increased insulin responsiveness Stuart et al. This view has been challenged by some recent studies where genetically manipulated mice Izumiya et al. It does seem important to consider that the contribution of the skeletal musculature to whole-body energy metabolism and substrate oxidation should not be based on the assessment of these parameters in a single muscle type.
Some of these effects correlate with observed shifts in muscle size and fibre type that occur with training Shaw et al. The pathways by which different fuels are oxidised to support tissue and cellular energy demands in animals are thoroughly dealt with in major textbooks and summarised in Fig.
The electrochemical proton gradient generated by the ETC then drives ATP synthesis via ATP synthase Fig. Because FAs are chemically more reduced molecules than carbohydrates, FAs are theoretically able to produce more energy when completely oxidised than an equivalent carbohydrate molecule.
In other words, the complete oxidation of six-carbon glucose consumes six oxygen molecules and produces six carbon dioxide molecules accompanied by the synthesis of 36 ATP molecules.
On the other hand, the complete oxidation of six-carbon hexanoic acid consumes eight oxygen molecules and produces six carbon dioxide molecules for 44 ATP molecules. Therefore, a switch to the oxidation of FAs as the major energy substrate should result in less efficient ATP production and an increase in whole-body energy expenditure that could lead to a loss of fat mass if energy intake remains constant Clapham abLeverve et al.
Pathways of substrate metabolism in muscle. Oxidation pathways of glucose, FAs and amino acids converge at the level of acetyl CoA. This proton motive force is dissipated by ATP synthesis and by proton leak via the adenine nucleotide transporter ANT and activated uncoupling proteins UCPx.
Demand for ATP and proton leak are greater determinants of oxygen consumption and heat production than the substrate being oxidised. Citation: Journal of Endocrinology2; Indirect calorimetry is often used in human and animal studies to determine total energy expenditure indirectly by the measurement of oxygen consumption and carbon dioxide productionand the measurement of oxygen consumption and carbon dioxide production can also be used to calculate the relative use of glucose and FAs to support that energy expenditure respiratory exchange ratio, RERassuming that any contribution of protein oxidation is relatively small and constant FerranniniArch et al.
However, in practice, it is unlikely that such theoretical calculations can be applied to the regulation of energy balance with any certainty. For instance, rarely does the measured RER shift from complete glucose oxidation 1. counter-ion transport and uncoupling protein activity Mazat et al.
The concepts of efficiency and plasticity in the coupling of substrate oxidation to energy conservation ATP synthesis have been expanded on in several authoritative review articles Harper et al.
In reality, coupling efficiency can vary significantly depending on changes in proton leak or ATP demand, but in cell systems at least, changes in substrate oxidation do not appear to influence the relationship between oxygen consumption and ATP synthesis Brand et al.
The above discussion clearly leads to the conclusion that the cost of generating mitochondrial ATP in terms of ETC activity and oxygen consumption can vary significantly and is not affected to any large extent by the substrate being oxidised to provide the reducing equivalents for electron transport.
Despite this, it is not uncommon to read about studies in whole animal systems particularly genetically modified mice where differences in fat mass are often mechanistically related to changes in the mRNA levels of FA metabolism genes in a variety of tissues without appropriate consideration of the contribution of these tissues to whole-body energy expenditure Abu-Elheiga et al.
For example, expression of oxygen consumption or heat production on a kilogram body weight basis can be misleading if animals have significantly different amounts of fat tissue, because the metabolic rate of fat per gram is much lower in tissues such as muscle and liver Frayn et al.
Similarly, the difference in daily food intake needed to contribute to a significant gain of body fat over several weeks in mice can be so small as to be undetectable unless large numbers of mice — are used for the comparison Tschop et al. Changes in the body weight and body fat of groups of adult mice with different genotypes on different diets should reflect cumulative differences in energy intake and energy expenditure.
However, any differences might not be easily detected if animals are assessed for food intake and energy expenditure individually in indirect calorimetry systems, away from their home cage and communal environment for only a 24—h period of the several weeks over which body weight and fat mass have been monitored.
AMP-activated protein kinase AMPK is recognised as a master regulator of energy metabolism, particularly in times of energy stress such as exercise, hypoxia and starvation Hardie et al. The activation of AMPK has been shown to acutely increase FA and glucose uptake and metabolism in a variety of experimental situations including in vitro and in vivo experiments in muscle Iglesias et al.
The long-term effects of AMPK activation in muscle lead to the activation of gene transcription pathways that increase mitochondrial biogenesis and proteins of oxidative metabolism Winder et al. The acute regulation of FA oxidation by AMPK is largely through the phosphorylation and inactivation of the enzyme acetyl CoA carboxylase 2 ACC2.
The pharmacological activation of AMPK has been shown to produce changes in muscle metabolic pathway capacity similar to those produced by exercise training O'Neill et al. A series of studies employing genetic deletion of Acc2 Acacb have reported reduced fat depots in association with increased FA oxidation in isolated muscle Abu-Elheiga et al.
These results suggest that the inhibition of ACC2 by the activation of AMPK or development of ACC2 inhibitors might promote FA oxidation and produce fat loss. Subsequent studies using independently generated Acc2 -knockout mice did not reproduce these effects, reporting that although these mice exhibited increased FA oxidation at the whole-body and isolated muscle level, there was no measurable difference in energy expenditure, fat mass or food intake Hoehn et al.
However, there was an increased glycogen content in muscle, an effect of AMPK activation noted previously Winder et al. Another study using independently generated genetically manipulated mice has reported no difference in body weight, food intake or fat mass in global or muscle-specific Acc2 gene-deleted mice Olson et al.
Therefore, it would appear that apart from theoretical calculations suggesting that increasing fat oxidation will drive increased energy expenditure, there is little experimental evidence to support the idea that energy expenditure can be increased simply by increasing substrate availability or by switching to oxidise FAs.
From an energy metabolism point of view, the flow of different substrates to tissues for oxidation or storage is largely under the control of the circulating hormone insulin.
After a meal, direct stimulation of the β-cells of the islets of Langerhans of the pancreas by nutrients glucose, FAs and amino acids increases insulin release into the circulation. Certain gut hormones GLP1 and GP can also augment insulin secretion, as can neural signals from the brain Thorens Insulin has many stimulatory and inhibitory actions in different tissues mediated by a complex intracellular signalling pathway, but for the purpose of this discussion, the actions of insulin to stimulate glucose uptake and metabolism in muscle and regulate FA metabolism will be a major focus.
The failure of insulin to appropriately regulate glucose and FA metabolism is termed insulin resistance, and this condition is most frequently observed in the muscle and liver of overweight or obese individuals Eckardt et al.
Insulin resistance is considered a significant predisposing factor for the development of type 2 diabetes T2D and therefore there is considerable research effort put into determining the mechanistic relationship between excess lipid accumulation obesity and insulin resistance, particularly in muscle.
Studies from over 20 years ago first showed that triglyceride accumulation in the muscle of high-fat diet-fed rats coincided with insulin resistance Storlien et al. Since then, the relationship between muscle lipid accumulation and insulin resistance has also been established in humans, and many mechanisms have been put forward to explain how lipid accumulation could generate insulin resistance Bosma et al.
Over the last decade, the major challenge has been determining whether these proposed mechanisms are universal or specific to the model of lipid-induced insulin resistance being studied.
It is also possible that different mechanisms are important at different times during the development of insulin resistance and that some proposed mechanisms depend on the experimental methods used to assess insulin action.
All discussions of the relationship between increased fat metabolism and insulin action are dependent on the methodology used to assess insulin resistance and the assumptions associated with different methodologies.
As has been mentioned previously, nearly all investigations of lipid-induced insulin resistance in rodent models utilise high-fat diet feeding to increase adiposity, but the methods of assessing insulin action can be quite varied and rely on glucose tolerance tests or insulin tolerance tests and less frequently because of the technical difficulty on hyperinsulinaemic—euglycaemic clamps.
Various technical considerations of glucose and insulin tolerance tests must be considered when discussing the metabolic implications of these tests for muscle insulin action. The timing and route of administration of glucose and duration of fast before glucose administration influence the results of glucose tolerance tests Andrikopoulos et al.
Insulin tolerance tests were devised largely to assess the effectiveness of counter-regulatory mechanisms in response to insulin-induced hypoglycaemia and therefore the utility of these tests to assess peripheral insulin action is debatable.
Neither glucose tolerance nor insulin tolerance tests give specific data regarding insulin effectiveness in muscle, although several methodological variations have used concurrent injection of radioactive tracers to assess glucose clearance into muscle during a glucose tolerance or insulin tolerance test Crosson et al.
The hyperinsulinaemic—euglycaemic clamp with glucose tracer administration gives the most reproducible assessment of muscle glucose clearance in response to constant insulin stimulation and constant glucose availability Ayala et al.
This technique relies on plasma insulin levels not insulin infusion rates during the comparison of the clamp being matched between the groups. In many studies, plasma insulin levels during the clamp are not reported, making the assessment of muscle insulin action difficult Chapman et al.
In vitro assessment of insulin effectiveness in isolated soleus or extensor digitorum longus muscle is also often used to demonstrate the effects of FA exposure Thompson et al.
reliance on diffusion and not on perfusion. While all the above methods can give useful information about the effects of muscle lipid accumulation on insulin action, this information can be specific for the test employed.
Even the data obtained from hyperinsulinaemic—euglycaemic clamp studies describe fluxes measured after at least an hour of exposure to constant insulin stimulation and constant glucose availability, a situation that is unlikely to ever exist in the normal h feeding—fasting cycle.
Therefore, it would seem important to consider the method used to demonstrate a difference in insulin action with lipid accumulation, when assessing the relevance of various mechanisms to reduced glucose metabolism in muscle when no restrictive experimental conditions e.
in vitro assessment, constant infusion and i. As has been mentioned above, the association between intramyocellular triglyceride IMTG content and insulin resistance is now well established in animals and obese humans, and most studies investigating the mechanisms of insulin resistance in muscle use high-fat diet rodent models.
It has also become standard practice in assessing the phenotype of genetically manipulated mice to place them on high-fat diets to investigate whether there is any impact favourable or detrimental of gene manipulation on glucose and energy homoeostasis.
There is a reasonable assumption that, independent of genetic background in animals or humans, overconsumption of energy-dense diets plays a major role in the accumulation of fats and development of metabolic derangements in muscle. In humans, overconsumption of energy-dense diets for a few weeks is enough to increase fat mass and have detrimental effects on whole-body insulin action Samocha-Bonet et al.
In mice, high-fat feeding for as little as a few days can impair glucose tolerance Turner et al. Studies that improved insulin sensitivity by low-calorie diets in patients with T2D were accompanied by a reduction in IMTG content Jazet et al.
Insulin resistance associated with ageing Nakagawa et al.
: Augmented fat metabolism efficiency| Nutrient Imbalance Provokes Mitochondrial Ros | However, other studies have not achieved similar effects. Tsiloulis and colleagues collected scWAT of obese men after 6 weeks of physical training and the mRNA levels of UCP1, CD, CITED, TBX1, LHX8, and TCF21 were not altered [ ]. Many factors may be involved in this diversity of results since the duration, frequency, and degree of intensity are associated with these effects. Thus, more human studies need to be conducted as many questions still need to be clarified. The fibroblast growth factor family FGF performs a range of cellular metabolic and physiological responses to maintain overall homeostasis. FGF 21 was first identified in mice and humans in 2, by Nishimura and colleagues through cDNA identification in different organs [ ]. While the gene in mice is located in chromosome 7 and encodes a preprotein of amino acids aa , in humans it is found in chromosome 19 and encodes a preprotein of aa. Most FGF family members have a high affinity to heparin sulfate, except for the endocrine FGF FGF subgroup, which consists of FGF 19 FGF 15 in rodents , FGF 21 e FGF 23 in humans [ ]. FGF molecules lack an extracellular heparin-binding domain and thus can enter the blood system [ ]. FGF 21 binds to a fibroblast growth factor tyrosine kinase receptor FGFR , which can be found in seven isoforms: 1b, 1c, 2b, 2c, 3b, 3c, and 4. The FGF 21 requires its dimerization with a klotho protein, called beta-klotho KLB. Thus, the FGFR-KLB receptors lead to the intracellular cascade that goes through the phosphorylation of FGFR substrate 2α FRS2α and the activation of Ras-MAPKs and PI3K-Akt kinases [ , , ]. Once FGF21 signaling requires KLB to activate FGFRs, the co-expression of these two receptors determines the sensitivity of a tissue or organ to FGF21 [ ]. FGF 21 is defined as a stress-responsive hormone [ ], which effect is subtle in physiological conditions but significantly exacerbated under nutritional, metabolic, oxidative, hormonal, or environmental challenges. FGF 21 is synthesized mainly in the liver and thymus but is also detected in skeletal muscle, pancreas, intestine, heart, β cells, and WAT and BAT [ ]. As an important metabolic regulator, acting mostly in glucose and lipid homeostasis, FGF 21 triggers lipolysis and FFAs released in circulation from WAT during prolonged fasting or starvation [ 29 , ]. PPAR-α is activated in the presence of FFA and improves FFA oxidation and ketone bodies formation for acting as energy sources during prolonged fasting. Thus, when PPAR-α activity increases, the production of FGF 21 in the liver also augments, leading to energy production, increased ketogenesis, gluconeogenesis, appetite, and systemic glucose uptake as adaptive responses to starvation [ ]. The activity of FGF 21 is not limited to starvation conditions, but it is also increased in adaptation to high-fat HF intake [ ]. Human studies inform that FGF21 production is stimulated in situations of decreased thermogenesis, reduction in adiponectin levels, and tissue breakdown markers, such as transaminases elevation mare than changes in levels of FFAs [ ]. Another means of increasing FGF21 levels, through PPAR-α activity, is through intense physical activity, growth hormone therapy, lactation, and milk ingestion in neonates [ , ]. Macronutrients such as proteins also regulate FGF 21 production through amino acid restriction [ ]. This process starts when the general control non-derepressible 2 GCN2 -eukaryotic initiation factor 2 eIF2 α pathway is activated inducing the binding of activating transcription factor 4 ATF4 to PGC-1 α [ , ]. After being secreted, its most important target is WAT, where FGF21 improves insulin sensitivity [ , ] and increments GLUT1 expression and consequently glucose uptake, as shown by in vitro 3T3-L1 adipocyte analyses [ , ]. The response element-binding protein ChREBP is sensitive to carbohydrates in the liver and ChREBP interaction with PPAR-γ in adipocytes modulates the expression of FGF In other words, the upregulation of ChREBP may induce the expression of this FGF [ ]. Another example of FGF 21 influence on carbohydrate metabolism is through the suppression of hepatic pyruvate dehydrogenase PD complex through PD kinase 4 activity [ ]. Additional transcription factors, such as retinoic acid RA receptor β RARβ , TRβ, cyclic AMP response element-binding protein H CREBH , RA receptor-related orphan receptor α RORα , respond to determinants in the liver and regulates FGF 21 production [ ]. WAT is not only a target of FGF21, but it is the major mediator of its effects. The processes of glucose- and insulin-sensitive responses depend on adiponectin production and secretion by this tissue [ ]. Adiponectin also reduces the levels of sphingolipid ceramides in obese animals, which have been associated with lipotoxicity [ ]. The action of FGF21 in WAT includes paracrine and autocrine actions and is mediated through the induction of PGC-1α protein in cold and through the enhanced levels of the thermogenic protein UCP1, which is a key protein for heat production [ ]. FGF 21 impact derives from increased PGC-1α levels and, consequently, expression of UCP1 [ ]. In conclusion, FGF 21 is involved in glucose uptake, lipogenesis, and lipolysis, depending on the metabolic state of the adipocytes. This dual phenomenon may depend on nutritional condition, FGF21 concentrations reached between pharmacological administration and physiological secretion [ ]. Thyroid Hormone TH is essential for metabolism in mammals and associates with many processes, including organism development, metabolic regulation, neural differentiation, and growth [ ]. TH is produced in the follicles of the thyroid gland and is synthesized through iodination of tyrosine residues in the glycoprotein thyroglobulin [ , ]. The main means of regulator its production is through thyroid-stimulating hormone TSH , which binds to the TSH receptor TSH-R expressed in the thyroid follicular cell basolateral membrane and is released by the anterior pituitary in response to a circulating TH [ ]. The biological response of TH is complex and highly regulated. It is mediated by thyroid hormone nuclear receptors TRs. The TR genes produce two main types of receptors, α and β, and their isoforms α1, α2, α3, β1, β2, and β3, but only α1, β1, β2, and β3 are T3-binding receptors, which are differentially expressed in tissues and have distinct roles in TH signaling [ , ]. TH enters the cell through membrane proteins monocarboxylate transporter 8 MCT8 and solute carrier organic anion transporter family member 1C1 9 OATP1C1 , then interacts with TR in the nucleus, which binds to the genomic thyroid-hormone responsive elements TREs and other nuclear proteins, including corepressors, coactivators, and cointegrators, leading to chromatin remodeling and the regulation of the UCP1 gene transcription [ , ]. This hormone is correlated with weight and energy expenditure. Thus, hypothyroidism, characterized by diminished TH levels, leads to hypometabolism, a condition associated with reduced resting energy expenditure, weight gain, high cholesterol levels, reduced lipolysis, and gluconeogenesis. On the other hand, hyperthyroidism, and elevated TH levels, induce a hypermetabolic state, characterized by increased resting energy expenditure, lower cholesterol levels, increased lipolysis and gluconeogenesis, and weight loss. Consequently, TH controls energy balance by regulating energy storage and expenditure regulating key metabolic pathways [ ]. TH regulates basal metabolic rate BMR through ATP production, used for metabolic processes, and by generating and maintaining ion gradient [ , , ]. TH maintains the BMR levels through the uncoupling oxidative phosphorylation in the mitochondria. When ATP production is compromised in skeletal muscle, TH increases the leak of protons through the mitochondrial inner membrane, stimulating more oxidation to maintain ATP synthesis [ ]. TH regulates metabolism primarily through actions in the brain, WAT, BAT, skeletal muscle, liver, and pancreas [ ]. This action, as already said, is through TH receptors TR isoforms, WAT has the adrenergic signaling increased by TRα [ ], otherwise BAT expresses TR α and β, as it needs TRα for adrenergic stimulation and TRβ for stimulating of UCP1, both for thermogenesis [ ]. TH regulates several aspects of lipid metabolism and human BAT from lipogenesis to lipoprotein signaling [ ]. Rats administrated with T3 showed how the central nervous system is important to the activation of BAT by TH through inhibition of hypothalamic AMP-activated protein kinase AMPK. Stimulation of sympathetic nervous system SNS activity leads to thermogenic gene expression in BAT [ ]. As discussed previously, β-AR is stimulated by NE in response to SNS [ 1 ]. The expression of UCP1, required for BAT thermogenesis, is regulated by NE and T3 synergistically, once the induction in separate is twofold, while combined is 20 -fold [ ]. Another way that UCP1 expression and thermogenesis are induced is through bile acid stimulation. G protein-coupled membrane bile acid receptor TGR5 is stimulated in BAT and results in D2 stimulation and local T3 production [ ]. In conclusion, several mechanisms have been proposed for the TH influence in the browning process, including cold exposure, adrenergic activation [ ], and bile acid signal [ ]. Thus, the stimulation of BAT activation and WAT browning increase the energy expenditure, loss of weight [ ], D2 activation, UCP1 level increase, and consequent thermogenesis [ ]. As previously discussed here, several exogenous factors are able to elicit browning of WAT and BAT activation. However, endogenous factors also play an important role in regulating the phenotype and physiology of these tissues. One of the most important endogenous factors that are related to the regulation of AT is the circadian rhythm, which is a refined system that acts as a master biological clock synchronizing daily and seasonal variations with the behavioral, cellular and tissue-autonomous clock, as well as several biological processes that include sleep—wake cycle, hormone secretion, lipid and glucose homeostasis, energy balance and body temperature [ ]. Disruption of circadian rhythm caused by aging, shift-work, irregular sleep, insomnia, or long exposure to light during the night is associated with sleep and metabolic disorders such as cardiovascular diseases, diabetes type 2 and obesity. Regarding metabolic diseases, AT plays a central role in metabolic and whole-body energy homeostasis, once its secretes several adipokines that regulate diverse processes in CNS and peripheral tissues. Leptin, a hormone mainly produced by adipocytes, is released into the circulation where it crosses the blood—brain barrier BBB , through a saturable system, and interact with its receptor in the hypothalamus LepRb [ , ]. Hsuchou and colleagues demonstrated that leptin signaling disruption through a pan-leptin receptor knockout POKO in mice was able to dysregulate feeding behavior, metabolic and circadian rhythm profile and thus promote an accentuating of obesity [ ]. Beyond control of feeding and metabolic processes, leptin also displays a role in energy balance through the increase of AT thermogenesis in BAT by sympathetic activation [ , ]. Recent studies have proposed that diurnal rhythm promotes differential modulation in activity, thermogenesis and fat oxidation in BAT. It was observed that plasmatic lipid metabolism was improved during daytime with a higher expression of lipoprotein lipase, FA uptake, and modulates lipid plasmatic concentration in BAT [ ]. In the same line, Matsushita and colleagues, assessed forty-four healthy men who received diet-induced thermogenesis DIT under room temperature 27 °C and cold 19 °C in the morning and in the evening by using 18 F-fluorodeoxy-D-glucose positron emission tomography. It was observed that thermogenic parameters presented better performance during the morning [ ]. Moreover, several studies have established that melatonin directly impacts BAT morphology and function, also, in a mechanism dependent on adrenergic activation mediated by NE release. Melatonin is related to an increase of BAT volume, and thermogenic capacity, associated with the increase of UCP1 mRNA expression and mitochondrial mass and functionality, as well as seric lipid concentration. These profiles are significatively impaired under melatonin deficiency but reverse with oral melatonin replacement [ , , ]. Growing evidence confirms the intimate relationship between circadian rhythm and AT, with emphasis on metabolic homeostasis and modulation of BAT activity. The characterization of how this process happens emerges as a strong diagnostic tool as well as a therapeutic approach concerning sleep disorders and metabolic diseases. Several studies suggest that food items can affect AT function. Curcumin stimulation was unable to induce the same effects in the epididymal WAT, though. This process was mediated by the NE-β3-AR pathway since the levels of NE and β3-AR were elevated in the inguinal WAT [ ]. Although studies are scarce regarding the impact of thyme in the WAT browning process, it was observed that 20 µM of thymol, a substance present in the essential oils of thyme, in the complete medium when placed in contact with 3T3-LI preadipocytes for 6—8 days was able to induce an increased gene and protein expression of the PGC-1α, PPARγ, and UCP1. Such increases were related to the activation of β3-AR, AMPK, PKA, and Mitogen-activated protein kinase p38 MAPK being accompanied by an increase in mitochondrial biogenesis [ ]. Cinnamon oil contains trans-cinnamic acid, which exposure to 3T3-L1 white adipocytes at µM high gene expression of Lhx8, Ppargc1, Prdm16, Ucp1, and Zic1 and markers of UCP1, PRDM16, and PGC-1α, indicating WAT browning [ ]. Quercetin, a flavonoid present in the onion, also proved to be efficient in the browning process since mice fed for 8 days with 0. Just as the combination of quercetin and resveratrol also induces the WAT browning phenotype [ ]. The resveratrol, present in the bark of grapes and other plants, also increases the expression of UCP-1, PRDM16, and PPARγ, suggesting that resveratrol induces the formation of beige adipocytes through the phosphorylation of AMPK, once treatment coupled with inhibition or the deletion of AMPK did not produce the same effects [ ]. The same was observed in the substances found in the mushroom and honey, which induced increased expression of brown fat markers via AMPK and PGC-1α [ ]. The peppers have capsaicin, an active compound responsible for the burning sensation that is also involved in the browning of WAT. The WT animals showed an increase in the expression of Ucp-1, Pgc-1α, Sirt-1, Prdm16, and exhibited browning of WAT via activation of the transient receptor potential vanilloid 1 TRPV1 , which is related to the synthesis of catecholamine or sirtuin 1 SIRT1 -mediated deacetylation of PPARγ, facilitating PPARγ-PRDM interaction. Other substances, such as carotenoids, are involved in the WAT browning process. Fucoxanthin, β-carotene, and citrus fruits are efficient in modulating the Ucp1 expression , , Another food component that is involved in the browning process of WAT is berberine, a molecule derived from the plants Coptis chinensis and Hydrastis canadensis. The group discovered berberine promotes BAT thermogenesis and WAT browning, since the igWAT, but not the epididymal, showed high levels of mRNA and UCP1 protein expression and increased mitochondrial biogenesis after injections. The brown adipocyte markers PGC-1α, CIDEA, Cox8b, and lsdp5 were also elevated and AMPK and PGC-1α are involved [ ]. In another study, the polyphenols from tea extracts 0. Another analysis with the extract induced In Magnolia Officinalis, two magnolol compounds 20 µM and Honokiol 1—20 µM when used to stimulate 3T3-L1 adipocytes increased protein levels of PGC-1α, PRDM16, and UCP-1 [ ]. Honokiol also increased protein expression levels of CIDEA, COX8, FGF21, PGC-1α, and UCP1 [ ]. The herb panax ginseng contains ginsenoside Rg1 10 μM of ginsenoside Rb1 , which is capable of considerably increasing the mRNA expression of UCP1, PGC-1α, and PRDM16 in mature 3T3-L1 adipocytes via PPARγ [ ], as well as activating the AMP-activated protein kinase pathway [ ]. The fish oil is rich in n-3 polyunsaturated fatty acids PUFAs , components that are associated with the formation of beige adipocytes, among them is eicosapentaenoic acid EPA. Mice fed different diets, including with EPA, for 8 weeks showed increased expression of β3-AR, PGC-1α, and UCP1 and exhibited high expression of PPAR [ ], though this effect is controversial since another animal study investigating a diet containing pure EPA 3. Docosahexaenoic acid DHA 1. However, knockout mice for TRPV1 did not achieve the same effect, showing that such events were mediated by SNS, TRPV1, and catecholamines [ ]. Conjugated linoleic acids CLAs also showed potential to induce browning process in the WAT [ ]. Once the overwhelming impact of infectious diseases has been alleviated by the development of efficient therapeutics, life expectancy has been continuously increasing World Health Organization, Age-associated diseases, including type 2 diabetes T2D , cardiovascular diseases CVDs , neurodegenerative pathologies, and obesity statistics are alarming and correlates with changes in the lifestyle of individuals throughout the world, including the diet, and impair the health spam rise. Western diets WDs are composed by food items enriched in processed sugar, white flour and salt and poor in fibers, vitamins and minerals [ ]. At the same time, the diet may be the remedy against the burden caused by these chronic diseases. While overnutrition often correlates with inflammatory and metabolic detrimental effects at molecular level, undernutrition without starvation presents many benefits. Calorie restriction CR and intermittent fasting IF are promising interventions against the overweight and obesity numbers, climbing specially in Western countries [ ]. CR, defined as reduced calorie consumption without malnutrition, is the best studied dietary intervention that increase health spam in experimental models. A plethora of human studies place CR as beneficial for expanding the health spam [ ]. These studies proceeded Weindruch and Sohal positive correlations between CR and health spam [ ] Click or tap here to enter text.. AT plasticity is one of the connections between CR and health benefits. Fabbiano and colleagues analyzed mice under CR and described that this regimen induces functional beige fat development in WAT, phenomenon that occur via enhanced type 2 immune response and SIRT1 expression in AT macrophages [ ]. The stress resistance provided by the IF practice places this regimen as a feasible dietary intervention against various devastating complex pathologies. Differently from CR, intermittent fasting IF does not influence the meal size, but decrease the number of meals in a given period [ ]. The fasting state leads to a metabolic switch, which increases the usage of free fatty acid FFA as energy source in comparison to glucose. In addition, IF favors the synthesis of ketone bodies KBs by the liver, molecules that act as an energy source during nutrient deprivation and induce a plethora of beneficial effects on the organism by acting upon the muscle, liver, heart, brain, intestine and AT [ , , ]. IF also impacts positively on AT remodeling. A DIO animal model submitted to repetitive fasting cycles displayed increased glucose tolerance, and diminished adipocyte hypertrophy and tissue inflammation [ ]. Mouse studies show that IF induces WAT mass decrease, elevation of AT UCP1 expression and thermogenic capacity [ , ], and augmented beige pre-adipocytes recruitment to WAT [ , , ] Fig. The impact of circadian rhythm and different diets on the WAT browning modulation. The secretion of melatonin, a circadian rhythm regulating neurohormone, is mediated by the release of Norepinephrine NE , which binds to β-adrenergic receptors. Adrenergic activation is one of the main mechanisms of WAT browning induction and BAT activation. Intermittent fasting IF associates with weight reduction, improved metabolic status due to increased glycemic tolerance, decreased white adipocyte hypertrophy and AT inflammation, and augmented expression of thermogenic genes such as UCP1 and recruitment of beige adipocytes. IF is also modulates the intestinal microbiome composition and diversity, a shift closely related to the induction of browning in the WAT. Caloric restriction CR is also associated with weight loss, promotes greater recruitment of beige adipocytes through the participation of M2 macrophage and eosinophil infiltration and in WAT. Finally, obesity-inducing diets correlate with increased lipid accumulation, WAT unhealthy expansion and dysregulation. Abnormal expansion of WAT promotes ER stress, greater induction of adipose cell apoptosis and inflammation through NF-κB transcription factor activation and increased pro-inflammatory cytokines secretion. An elegant study conducted by Li and colleagues informed that mice under IF cycles display an intestinal microbiome composition shift associated with increased levels of the fermentation products lactate and acetate. They also show that the modulation of the gut microbiota by IF is crucial for its browning effect, as microbiota-depleted mice present impaired IF-induced AT beiging and fecal microbiota transfer from these mice to antibiotics treated animals display increased browning of WAT [ ] Fig. Unexpectedly, a human study conducted by von Schwartzenberg and colleagues showed that CR may diminish bacterial abundance, deeply change gut microbiome composition and diversity, impair nutrient absorption, and favor the outgrowth of the pathobiont Clostridioides difficile. This diet also led to a decrease in bile acid BA levels [ ]. BA, nonesterified fatty acids, are synthesized during the browning of WAT, a phenomena associated with the potentiation of the lipolytic machinery [ ]. These fatty acids can not only activate UCP-1 allosterically, but also serve as fuel for oxidative phosphorylation and consequently heat generation in BAT [ 1 ]. Furthermore, in the liver they are used for the generation of acylcarnitines and VLDL which is used as source for thermogenesis [ ]. Moreover, studies show that the increase in brite and brown adipocytes in WAT leads to an elevation in lipoprotein lipase LPL activity and subsequently an increase in circulating lipids available for BAT through intravascular hydrolysis of chylomicron triglycerides [ ]. Consequently, these mechanisms result in the generation of cholesterol-enriched lipoprotein remnants, which upon activation of BAT accelerates the flow of cholesterol to the liver [ ]. BA are steroid acids derived from dietary cholesterol catabolism. These acids are synthesized in the liver and act to aid digestion and absorption of fat in the intestine, in addition to playing an essential role in lipid metabolism. BA act in other tissues, such as AT, as signaling molecules through interaction with the nuclear Farnesoid X receptor FXR and the G protein-coupled membrane receptor TGR5 [ ]. Recent studies have shown that BA play a relevant role in BAT activation and increased thermogenesis in adipocytes. In rodents, the activation of BAT by BA is dependent on its interaction with the TGR5 receptor and expression of the enzyme type 2 iodothyronine deiodinase DIO2. Additionally, experiments with oral supplementation of BA in humans indicated increased BAT activity in humans [ , ]. Another experiment performed under thermoneutrality, demonstrated an improvement in glycemic metabolism and lipogenesis in the liver and fat accumulation in the TA and also induced an improvement in thermogenic parameters and mitigation of the impact of diet-induced obesity after feeding mice with HFD associated with BA [ ]. Moreover, BAT activation also promotes liver protection. In a study performed with animals under alcohol-induced hepatic steatosis or liver injury, activation of the TGR5 receptor induced improvement of clinical condition. The increase in thermogenesis in BAT promotes an increase in lipid metabolism with lower availability of circulating FFA and, consequently, lower absorption of these molecules by the liver [ ]. However, if on the one hand BAs have been shown to be effective in inducing browning, on the other hand the excess of these acids is capable of promoting an antagonistic effect, such as mitochondrial dysfunction and expression of genes associated with cellular senescence in adipose cells [ ]. ATs are embryologically distinct from other tissues and are formed according to specific stimuli during embryo development, including Bone morphogenetic proteins BMPs , pleiotropic molecules that interact with type I and type II BMP receptors and influence embryogenesis [ , ]. Noteworthy, BMP4 overexpression was found to increase UCP1 and other beiging markers, as Hoxc9, Tbx1, and Tbx15 [ ]. These different phenotypes induced by the BMPs, including the induced beige adipocytes, highlight how relevant transcriptional regulation is for determining the cells functions and characteristics. The main proteins that regulate gene expression are the transcriptional factors TFs , DNA binding proteins that modulate gene transcription by interacting with the gene promoter or cis-regulatory elements, such as enhancers and silencers, and include PPAR proteins, PGC-1α, and PRDM16 [ , ]. In addition to its roles in ATs development [ ], PPARγ is a central TF for adipogenesis and lipid storage regulation, influences cell thermogenic capacity, and impacts lipid metabolism and insulin sensitivity [ ]. This TF is expressed in elevated levels in ATs [ ], and upon ligand binding PPARγ recruits different cofactor sets for controlling the expression of specific genes. The use of PPARγ full agonists is associated with improved insulin sensitivity and induces WAT browning, but can cause detrimental effects, such as undesirable weight gain and augmented visceral adiposity [ ]. PPARα acts synergistically with PPARγ in inducing robust WAT browning in vivo [ ], and is currently considered a prominent target for treating metabolic disorders [ ]. A way that PPAR agonists provoke WAT browning is by stabilizing PRDM16 [ ], a protein that activates a complete set of thermogenic genes in WAT [ ]. PRDM16 is essential for browning particularly in scWAT, once its induction in visceral depots does not correlate with thermogenesis [ ]. Mice lacking Prdm16 in scWAT are unable to induce browning within subcutaneous depots after stimuli [ ]. Ectopic PRDM16 expression induce thermogenic genes in several cell types [ ]. PRDM16 AT overexpression in rodents copes with augmented energy expenditure and DIO resistance [ ] PRDM16 acts by binding to specific regulatory sequences in DNA and by interacting with other proteins [ ], such as PGC-1α [ ]. PGC-1α plays a key role in the adapting thermogenesis. First described in cold-induced adaptive thermogenesis analyses [ ], this transcriptional coactivator participates in the regulation of a plethora of cellular functions, including mitochondrial biogenesis, oxidative phosphorylation, and gluconeogenesis [ , ]. Once PGC-1α influences genes related to energy metabolism, it is expressed mostly in tissues that require an elevated amount of energy, like AT, liver, skeletal muscle, and brain [ ]. When overexpressed, Pgc1α induces mitochondrial biogenesis [ ]. Another key regulator of the browning process is CIDEA. Initially described as a mitochondrial protein, CIDEA was further discovered to be associated with cell lipid droplets LD [ , , ]. This molecule leads to the occurrence of browning by inhibiting the suppression of UCP1 gene expression mediated by liver-X receptors LXRs and increasing PPARγ binding strength to the UCP1 enhancer [ ]. As detailed in this review, UCP1 is found in the inner mitochondrial membrane and acts by uncoupling the electron transport chain and oxidative phosphorylation, releasing energy as heat [ 1 ]. The existence of molecular markers for the browning process can be useful for investigating AT plasticity status and correlate with health and disease. Zfp is a TF that directly binds to the UCP1 and PGC1α promoters and induce WAT browning upon cold exposure. Zfp overexpression copes with augmented multilocular lipid droplets LDs biogenesis and increased oxygen consumption and UCP1 levels [ ]. HSF1 was described to cooperate with PGC1α in igWAT, favoring the induction of the thermogenic and mitochondrial gene programs, which leads to augmented energy consumption. HSF1-deficient mice are cold intolerant due to decreased β oxidation and UCP1 expression. HSF1 activation associates with scWAT browning, non-shivering thermogenesis and energy consumption [ , ]. IRF4 also cooperates with PGC1α and was described to inhibit lipogenesis in adipocytes [ ]. While IRF4 overexpression favors beiging in epididymal WAT, the absence of this factor is linked to diminished energy expenditure and increased risk to hypothermia [ ]. The TF NFIA presents a crucial role in the initial steps of thermogenic gene regulation, as its increased levels in thermogenic adipocytes precedes PPARγ upregulation in these cells [ ]. The TF EBF2 uncouples adipocyte mitochondrial respiration and is sufficient for WAT browning. Increased EBF2 levels in WAT leads to activation of the thermogenic program, favoring increased oxygen consumption and resistance for weight gain. EBF2-KO mice show impaired WAT browning and ablates the brown fat-specific characteristics of BAT [ , , ]. EBF2 activity is regulated by the action of ZFP, which binds to EBF2 and recruits NuRD nucleosome remodeling deacetylase corepressor complex to suppress EBF2 activity in thermogenic genes regulatory sequences [ ]. Absence of ZFP associates with PPARγ binding to thermogenic gene enhancers, WAT browning and non-shivering thermogenesis [ ]. The protein transducin-like enhancer of split 3 TLE3 was first described by Villanueva and colleagues to increase PPARγ adipogenic activity [ ]. Deletion of TLE3 copes with increased energy expenditure and mitochondrial oxidative metabolism in adipocytes, characteristics associated with the browning process [ ] KLF11, TAF7L, ZBTB16, EWS, PLAC8, ERRα, ΕRRγ and other TFs that were described to promote WAT browning. In contrast, FOXO1, TWIST1, p, LXRα, pRB, RIP, REVERBα acting repressing AT beiging by impacting on the activity of EBF2, PRDM16, PGC1α and other activating TF [ , , ]. In order the transcription to occur, chromatin needs to be accessible to polymerases and TFs. Remodeling enzymes, which are classified in covalent histone modifiers and ATP-dependent chromatin remodeling complexes, actively modify chromatin status in response to environmental cues [ ]. Histone covalent modifiers are enzymes that chemically modify positively-charged amino acids mainly lysine present in these proteins. Histone acetyltransferases HATs and histone deacetylases HDACs catalyze the removal or insertion of acetyl group, respectively, influencing histone acetylation pattern [ ]. The HAT CBP was shown to inhibit the browning process, once it is key in white adipocyte differentiation [ ]. Histone acetylation pattern has been described to present a critical role in adipocyte identity, as cold exposure leads beige adipocytes to show histone acetylation pattern associated with brown phenotype [ ]. HDACs catalyze the deacetylation of amino acid residues in histones, reactions favor gene repression. These enzymes are categorized into four groups, namely class I HDAC1—3 and 8 , class IIa HDAC4, 5, 7, and 9 , class IIb HDAC6 and 10 , class III SIRT1—7 , and class IV HDAC11 [ ]. HDAC1 expression is augmented in WAT and copes with decreased levels of proteins associated with non-shivering thermogenesis, as UCP1 and PPARγ [ ]. HDAC3 levels also suppress WAT browning, as absence in WAT correlates with H3K27ac on enhancers of Ucp1 and Ppar g , signature associated with tissue improved oxidative capacity, mitochondrial biogenesis, and thermogenesis [ ]. Experiments involving the deletion of HDAC9 shows that this enzyme also contributes for the metabolic dysfunction characteristic of HFD-fed rodents [ ]. HDAC11 is another element that impairs WAT beiging, once its removal favors at thermogenesis in diet-induced obese DIO mice [ ]. Other key HDAC is the SIRT family SIRT1, SIRT2, SIRT3, SIRT5, and SIRT6. SIRT1 deletion in HFD-treated mice is associated with diminished amounts of PGC-1α, FGF21, and UCP1 in epididymal WAT eWAT [ ]. Histones can also be post-translationally modified by histone methyltransferases HMTs and histone demethylases, which influence gene activation or suppression depending on the modified residue position and valency [ ]. There are two categories of HMTs: lysine methyltransferases KMTs and arginine methyltransferases RMTs. Rodent studies show that inactivation of the KMT MLL3 favors improved insulin sensitivity and augmented energy expenditure [ ]. Absence of another KMT, the EHMT1, was shown to impair the thermogenic program in WAT [ ]. Histone demethylases HDM catalyze histone demethylation processes and are also classified according to the characteristics of the modified amino acid. The KDM LSD1 expression, which was found to be induced by chronic cold exposure and β3-adrenergic stimulation, leads to increased mitochondrial activity in WAT by cooperating with NRF1 [ , , ]. In addition, rodents presenting increased LSD1 levels are associated with igWAT browning in lean animals and with lower weight gain in the context of DIO [ ] Also induced by β3-adrenergic stimulation, the KDM3A JMJD1A directly regulates ppar a and ucp1 genes and was found to be crucial for WAT browning [ ]. Specific DNA sequences called CpG islands can also be methylated for gene transcription regulation by DNA methyltransferases DNMTs , which switches off gene expression. Ten-eleven translocation TET family enzymes switch on the gene expression by demethylation [ ]. Gene transcription can also be impacted by the action of ATP-dependent chromatin remodeling complexes, which, differently from the covalent histone modifiers, alter the interaction between the DNA and the nucleosome non-covalently using ATP hydrolysis as an energy source. Abe and colleagues suggested that BRG1 is necessary for thermogenesis induction [ ]. Other key actors in gene transcription regulation are the microRNAs MiR , small non-coding regulatory ribonucleic acids maximum nucleotides that influence gene expression post transcriptionally. These elements regulate gene expression by several mechanisms, including binding to mRNA strands to repress protein translation and to favor decapping, deadenylation and ultimately degradation of target mRNA in P-bodies, and direct translation inhibition. However, many studies suggest that miRNAs are also capable of activating transcription protein translation, fact that highlights miRNAs as central participants in the fine regulation of gene expression in response to specific stimuli [ ]. Raymond and others described that miR inhibition copes with impaired WAT browning [ ]. Another miRNA that influences WAT homeostasis is miR, which deletion leads to WAT enlargement and overexpression inhibits the progression of obesity in the DIO in mice [ ]. In contrast, miR, miR, and miR inhibit TFs associated with the browning process [ 61 , 63 , ]. Fu and colleagues showed that miRa inhibits WAT beiging via FGF21 [ ], miRNA, miRNA Let-7i-5p and miRb- 5p are molecules that impair WAT browning process, which inhibition promotes beiging [ , , ] Fig. Other small non-coding RNAs sncRNAs impact on gene expression and are extensively reviewed elsewhere. WAT browning transcriptional regulation. The transcriptional regulation of the WAT beiging process involves the action of a plethora of specific proteins and nucleic acids. This orchestra of trans-acting factors modulates genes associated with oxidative capacity, mitochondrial biogenesis and non-shivering thermogenesis. While the action of some factors, such as PGC-1α, IRF4, PRDM16, ZFP, EHMT1 and RNAs, copes with WAT browning, TLE3, ZFP, and the NuRD complex, another set of RNAs e others inhibit this process, favoring adipogenesis and white adipocyte differentiation. Non-coding RNAs that present average length longer than nucleotides are called LncRNAs [ , ] These nucleic acids have been described to influence gene transcription through several mechanisms, including the induction of more efficient protein translation by binding to an internal ribosome entry site IRES , increase of mRNAs stability, polyubiquitination process inhibition thus increasing protein stability, binding to specific proteins in the cytoplasm, and acting as a sponge sequestering miRNAs [ , , , , ]. Zhao and collaborators identified lncRNA 1 Blnc1 as a driver for beige adipocyte differentiation and WAT browning [ ]. LncRNAs also interact with other TFs, such as PGC-1α, ZFP, and PRDM16 for full transcription [ , , ]. PRDM16, a coregulator of PPAR, cooperates with EHMT1 for WAT browning induction. As EHMT1, other covalent histone modifiers form complexes with ATP-dependent chromatin-remodelers [ ]. The chromatin-remodelers BRG1 and the BAF also interact with the TF EBF2 for gene transcription [ , ]. Noteworthy, AT functional and morphological plasticity is represented by the extreme dynamic beige adipocyte chromatin state. Beige adipocytes show a chromatin signature similar to the pattern presented by brown adipocytes after cold exposure and display a chromatin signature associated with white adipocytes after re-warming to 30 °C. However, if the beige adipocytes were cold-induced, these cells displayed epigenetic marks that favored rapid thermogenic genes expression upon β-adrenergic stimulation, even after temperature rise, suggesting the occurrence of epigenomic memory [ , ]. For the past decades, the browning process has been deeply explored for managing both metabolic syndromes, and hypermetabolic diseases. Proposing treatment, the cold was the pioneer mechanism associated with a great elicitation of this event in mice. However, the applicability and outcomes of this approach in humans are far from exciting. Technological advances provide efficient mechanisms that can be employed to improve a biological system. Recent studies applied bioengineers to achieve the improvement of browning in adipose tissue. The injection of M2 AT macrophages ATMs from transgenic mwRIPKD mice, a specific knockdown of RIP that is related to activation of M1 polarization, in HFD-fed obese wild type mice could recover the disruption induced by obesity through browning induction [ ]. WAT-derived stem cells ASCs from humans and rats could be differentiated into BAT under browning conditions in three-dimensional 3D polyethylene glycol PEG hydrogel [ ]. In addition to bioengineering, a field that has been explored is the transplantation or re-implantation of BAT, also termed ex-BAT, to increase this endogenous tissue. The harvested scWAT is differentiated into brite and then is re-implanted in the same area promoting the local increase of BAT, displaying great outcomes [ ]. Aiming for a less invasive procedure, the use of a microneedle patch to address the browning agents directly to the scWAT for inducing browning of this tissue is another strategy investigated presently [ ]. These strategies aim to promote the increase of endogenous BAT, as well as its activity with an applicable method, reducing the degree of invasiveness and risks of rejection by the body. Several studies have targeted increasing BAT mass and activity, but when the aim is inhibiting the browning process to avoid a worsening prognosis, what is proposed? Expanding knowledge of the pathways involved is the main way of inhibiting that event. It is well established the role of parathyroid hormone PTH in the elicitation of browning. In the tumor context, it was identified as a peptide-derived tumor, parathyroid hormone-related protein PTHrP that favors browning and cachexia [ 16 ]. Blocking PTHR specifically in ATs inhibits browning and wasting of this tissue, as well preserves muscle integrity, and ameliorates muscle-related strength, protecting mice from cachexia [ ]. Currently, some pharmacological approaches have displayed alternative and prominent ways to restrain browning. Metformin demonstrated to be efficient to prevent the browning of the scWAT, as well as the inhibition of mevalonate pathways by the use of Statin or Fluvastatin, which implicates in the disruption of browning [ , ]. However, further studies should be conducted to promote a broader and more detailed debate on the topic. Given the data presented, it is evident that the benefits and dangers associated with activation or inhibition of the browning process are closely linked to the type of disorders displayed in the individual's body at the metabolic, cellular, or physiological level. Taking as criteria patients with metabolic syndromes and obesity, browning process emerges as a promising therapeutic approach, mainly due to its several benefits, such as improvement of clinical conditions associated with lower side effects, and the multi-stimulatory character, which allows numerous safe and feasible ways of induction. In contrast, the ways and means of inhibiting browning to prevent the development of comorbidities in cases of chronic or acute hypermetabolism are still poorly explored. In this way, browning process needs to be deeply explored and can be used as a key factor in different therapeutical approaches in health and diseases. Emerging insights into the metabolic and immunological role of browning of the white adipose tissue are also discussed, along with the developments that can be expected from these promising targets for therapy of metabolic and chronic disease in the forthcoming future. A major discussion pointed by the scientific community about the investigation of AT in mice is how much the results obtained in the animal model would reproduce the anatomorphophysiologies of these tissues in humans, since, unlike humans, mice have a significant amount of BAT during embryonic and adult, as well as they differ in several other factors, such as expression of molecular markers, activation profile and location. Human BAT was discovered to be more similar to beige compared to classical BAT markers [ , ]. Another relevant point concerns the fact that the BAT deposits most used for research purposes are the BAT located in the interscapular region iBAT , while in humans there is a greater abundance of this tissue in the clavicular and neck regions, presenting compositional differences that represent obstacles in the overlapping of scientific findings. In this sense, Mo and colleagues identified an analogous deposit of BAT in mouse embryos, which is maintained during adulthood, and in humans called supraclavicular BAT scBAT. The scBAT shows similarities to scBAT in humans in terms of location, morphology and thermogenic capacity [ ]. In which the authors observed a greater similarity between several cellular and molecular parameters between the BAT of humans and mice [ ]. The technique generated controversies that were discussed by Kajimura and Spiegelman and replicated by the authors of the original article , Current research place WAT browning as an extremely dynamic process that is influenced by several factors , including temperature, physical exercising, thyroid hormones, circardian rhythm, food components and dietary regimens. The participation of AT plasticity on the organism metabolic health and inflammatory status spot this process as a promising therapeutic target for decreasing the risk associated with many chronic diseases. Further efforts in investigating AT plasticity must alleviate the burden of these devastating life-style associated pathologies. Cannon B, Nedergaard J. Brown adipose tissue: function and physiological significance. Physiol Rev. Article CAS PubMed Google Scholar. Moriya K, Arnold J, LeBlanc J. Shivering and nonshivering thermogenesis in exercised cold-deacclimated rats. Eur J Appl Physiol Occup Physiol. Prunet-Marcassus B, Cousin B, Caton D, André M, Pénicaud L, Casteilla L. From heterogeneity to plasticity in adipose tissues: site-specific differences. Exp Cell Res. Barbatelli G, Murano I, Madsen L, Hao Q, Jimenez M, Kristiansen K, et al. The emergence of cold-induced brown adipocytes in mouse white fat depots is determined predominantly by white to brown adipocyte transdifferentiation. Am J Physiol Metab. CAS Google Scholar. Suárez-Zamorano N, Fabbiano S, Chevalier C, Stojanović O, Colin DJ, Stevanović A, et al. Microbiota depletion promotes browning of white adipose tissue and reduces obesity. Nat Med. Article PubMed PubMed Central CAS Google Scholar. Johann K, Cremer AL, Fischer AW, Heine M, Pensado ER, Resch J, et al. Thyroid-hormone-induced browning of white adipose tissue does not contribute to thermogenesis and glucose consumption. Cell Rep. Barberá MJ, Schlüter A, Pedraza N, Iglesias R, Villarroya F, Giralt M. Peroxisome proliferator-activated receptor α activates transcription of the brown fat uncoupling protein-1 gene: a link between regulation of the thermogenic and lipid oxidation pathways in the brown fat cell. J Biol Chem. Article PubMed Google Scholar. Schulz TJ, Huang TL, Tran TT, Zhang H, Townsend KL, Shadrach JL, et al. Identification of inducible brown adipocyte progenitors residing in skeletal muscle and white fat. Proc Natl Acad Sci U S A. Villarroya F, Peyrou M, Giralt M. Transcriptional regulation of the uncoupling protein-1 gene. Kalinovich AV, de Jong JMA, Cannon B, Nedergaard J. UCP1 in adipose tissues: two steps to full browning. Yau WW, Singh BK, Lesmana R, Zhou J, Sinha RA, Wong KA, et al. Thyroid hormone T 3 stimulates brown adipose tissue activation via mitochondrial biogenesis and MTOR-mediated mitophagy. Pérez-Martí A, Garcia-Guasch M, Tresserra-Rimbau A, Carrilho-Do-Rosário A, Estruch R, Salas-Salvadó J, et al. A low-protein diet induces body weight loss and browning of subcutaneous white adipose tissue through enhanced expression of hepatic fibroblast growth factor 21 FGF Mol Nutr Food Res. Article CAS Google Scholar. Finlin BS, Memetimin H, Confides AL, Kasza I, Zhu B, Vekaria HJ, et al. Human adipose beiging in response to cold and mirabegron. JCI Insight. Article PubMed Central Google Scholar. Otero-Díaz B, Rodríguez-Flores M, Sánchez-Muñoz V, Monraz-Preciado F, Ordoñez-Ortega S, Becerril-Elias V, et al. Exercise induces white adipose tissue browning across the weight spectrum in humans. Front Physiol. Article PubMed PubMed Central Google Scholar. Carrière A, Jeanson Y, Berger-Müller S, André M, Chenouard V, Arnaud E, et al. Browning of white adipose cells by intermediate metabolites: an adaptive mechanism to alleviate redox pressure. Article PubMed CAS Google Scholar. Kir S, White JP, Kleiner S, Kazak L, Cohen P, Baracos VE, et al. Tumour-derived PTH-related protein triggers adipose tissue browning and cancer cachexia. Article CAS PubMed PubMed Central Google Scholar. Abdullahi A, Samadi O, Auger C, Kanagalingam T, Boehning D, Bi S, et al. Browning of white adipose tissue after a burn injury promotes hepatic steatosis and dysfunction. Cell Death Dis. Lucchini FC, Wueest S, Challa TD, Item F, Modica S, Borsigova M, et al. ASK1 inhibits browning of white adipose tissue in obesity. Nat Commun. Frontini A, Cinti S. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab. Luo L, Liu M. Adipose tissue in control of metabolism. J Endocrinol. Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. Andrade-Oliveira V, Câmara NOS, Moraes-Vieira PM. Adipokines as drug targets in diabetes and underlying disturbances. J Diabetes Res. Article Google Scholar. Jokinen R, Pirnes-karhu S, Pietiläinen KH, Pirinen E. Redox Biol. Parimisetty A, Dorsemans AC, Awada R, Ravanan P, Diotel N, Lefebvre C. Secret talk between adipose tissue and central nervous system via secreted factors-an emerging frontier in the neurodegenerative research. J Neuroinflam. Reilly SM, Saltiel AR. Adapting to obesity with adipose tissue inflammation. Nat Rev Endocrinol. Park A. Distinction of white, beige and brown adipocytes derived from mesenchymal stem cells. World J Stem Cells. Dalal S. Lipid metabolism in cancer cachexia. Ann Palliat Med. Wu J, Boström P, Sparks LM, Ye L, Choi JH, Giang AH, et al. Beige adipocytes are a distinct type of thermogenic fat cell in mouse and human. Cuevas-Ramos D, Mehta R, Aguilar-Salinas CA. Fibroblast growth factor 21 and browning of white adipose tissue. Bargut TCL, Souza-Mello V, Aguila MB, Mandarim-de-Lacerda CA. Browning of white adipose tissue: lessons from experimental models. Horm Mol Biol Clin Investig. Shao M, Wang QA, Song A, Vishvanath L, Busbuso NC, Scherer PE, et al. Cellular origins of beige fat cells revisited. Vargas-Castillo A, Fuentes-Romero R, Rodriguez-Lopez LA, Torres N, Tovar AR. Understanding the biology of thermogenic fat: is browning a new approach to the treatment of obesity? Arch Med Res. Long JZ, Svensson KJ, Tsai L, Zeng X, Roh HC, Kong X, et al. A smooth muscle-like origin for beige adipocytes. Shao M, Vishvanath L, Busbuso NC, Hepler C, Shan B, Sharma AX, et al. Oguri Y, Shinoda K, Kim H, Alba DL, Bolus WR, Wang Q, et al. CD81 controls beige fat progenitor cell growth and energy balance via FAK signaling. Tabuchi C, Sul HS. Signaling pathways regulating thermogenesis. Front Endocrinol Lausanne. Google Scholar. Himms-hagen J, Melnyk A, Zingaretti MC, Ceresi E, Barbatelli G, Cinti S. Multilocular fat cells in WAT of CLtreated rats derive directly from white adipocytes. Am J Physiol Cell Physiol. Golozoubova V, Cannon B, Nedergaard J. UCP1 is essential for adaptive adrenergic nonshivering thermogenesis. Am J Physiol - Endocrinol Metab. Schenk H, Franke H, Heim T. Structure and function of brown adipose tissue. Acta Histochem. Klingenberg M, Echtay KS, Bienengraeber M, Winkler E, Huang SG. Structure-function relationship in UCP1. Int J Obes. Ricquier D, Bouillaud F. The uncoupling protein homologues: UCP1, UCP2, UCP3 StUCP and AtUCP. Biochem J. Echtay KS. Mitochondrial uncoupling proteins—What is their physiological role? Free Radic Biol Med. Heaton JM. The distribution of brown adipose tissue in the human. J Anat. CAS PubMed PubMed Central Google Scholar. Leitner BP, Huang S, Brychta RJ, Duckworth CJ, Baskin AS, McGehee S, et al. Mapping of human brown adipose tissue in lean and obese young men. Proc Natl Acad Sci USA. Klingenberg M, Echtay KS. Uncoupling proteins: the issues from a biochemist point of view. Biochim Biophys Acta Bioenerg. Chechi K, Grundberg E, Richard D, Chechi K, Vijay J, Voisine P, et al. Hoang T, Smith MD, Jelokhani-Niaraki M. Expression, folding, and proton transport activity of human uncoupling protein-1 ucp1 in lipid membranes. Ježek P, Jabůrek M, Porter RK. Uncoupling mechanism and redox regulation of mitochondrial uncoupling protein 1 UCP1. Macher G, Koehler M, Rupprecht A, Kreiter J, Hinterdorfer P, Pohl EE. Inhibition of mitochondrial UCP1 and UCP3 by purine nucleotides and phosphate. Biochim Biophys Acta Biomembr. Kozak UC, Kopecky J, Teisinger J, Enerbäck S, Boyer B, Kozak LP. An upstream enhancer regulating brown-fat-specific expression of the mitochondrial uncoupling protein gene. Mol Cell Biol. Yubero P, Barbera MJ, Alvarez R, Vinas O, Mampel T, Iglesias R, et al. Dominant negative regulation by c-Jun of transcription of the uncoupling protein-1 gene through a proximal cAMP-regulatory element: a mechanism for repressing basal and norepinephrine-induced expression of the gene before brown adipocyte differentiation. Mol Endocrinol. Manchado C, Yubero P, Vinas O, Iglesias R, Villarroya F, Mampel T, et al. Cassard-Doulcier AM, Gelly C, Bouillaud F, Ricquier D. A bp enhancer of the rat uncoupling protein-1 UCP-1 gene controls specific and regulated expression in brown adipose tissue. Sears IB, MacGinnitie MA, Kovacs LG, Graves RA. Differentiation-dependent expression of the brown adipocyte uncoupling protein gene: regulation by peroxisome proliferator-activated receptor gamma. Wang H, Zhang Y, Yehuda-Shnaidman E, Medvedev AV, Kumar N, Daniel KW, et al. Liver X receptor α is a transcriptional repressor of the uncoupling protein 1 gene and the brown fat phenotype. Kiskinis E, Hallberg M, Christian M, Olofsson M, Dilworth SM, White R, et al. RIP directs histone and DNA methylation to silence Ucp1 expression in white adipocytes. EMBO J. Shore A, Karamitri A, Kemp P, Speakman JR, Lomax MA. Role of Ucp1 enhancer methylation and chromatin remodelling in the control of Ucp1 expression in murine adipose tissue. Gebert LFR, MacRae IJ. Regulation of microRNA function in animals. Nat Rev Mol Cell Biol. Oliverio M, Schmidt E, Mauer J, Baitzel C, Hansmeier N, Khani S, et al. Dicer1-miRBace1 signalling controls brown adipose tissue differentiation and function. Nat Cell Biol. Zhang H, Guan M, Townsend KL, Huang TL, An D, Yan X, et al. Micro RNA regulates brown adipogenesis via a novel HIF 1an- AMPK - PGC 1α signaling network. Mitochondrial metabolism is often altered in inherited diseases, such as inborn errors of metabolism IEMs that impinge upon ROS generation. Inhibition of OXPHOS increases ROS generation due to a backlog of electrons in the various complexes, resulting in electron leak, ROS generation, and production of H 2 O 2. In IEMs affecting the ETC or other pathways of ATP generation, increased oxidative stress is often observed, while the exact mechanisms for increased ROS production are unknown. It is hypothesized that mutations affecting the formation of the protein complexes in the ETC or mutations that modify their assembly increase ROS generation by facilitating electron leak Olsen et al. Additionally, accumulation of toxic intermediates, often observed in IEMs, can increase the ROS generation by further decreasing OXPHOS activity, as in the case of medium-chain acyl-CoA dehydrogenase MCAD deficiency. MCAD deficiency reflects the accumulation of medium-chain fatty acid derivatives, including cisdecenoic acid, octanoate, and decanoate, with these metabolites altering levels of antioxidants and increasing markers of oxidative stress Schuck et al. Intriguingly, IEMs display metabolic reprograming with a switch to glycolysis for both ATP production and muted ROS generation Olsen et al. Specifically, in myoclonic epilepsy with ragged red fibers MERRF , increased intracellular H 2 O 2 levels correspond with increased AMPK phosphorylation and expression of GLUT1, hexokinase II, and lactate dehydrogenase. These results, as well as increased lactic acid production, all point to increased glycolysis De la Mata et al. In multiple acyl-CoA dehydrogenase deficiency MADD , mutations in ETFa , ETFb , or ETFDH , lead to decreased ATP production with an accumulation of organic acids, including glutaric acid as well as acyl-carnitines. A subset of these patients is riboflavin responsive RR-MADD with high dose riboflavin alleviating some symptoms. Similar to MERRF, many RR-MADD patients exhibit increased oxidative stress Cornelius et al. This defect may be due to defective electron transfer and increased electron leak from the misfolded ETFDH protein and decreased binding of CoQ10 Cornelius et al. Treatment with CoQ10, but not riboflavin, decreased ROS levels Cornelius et al. Analysis of mitochondrial function from RR-MADD fibroblasts showed increased mitochondrial fragmentation and reduced β-oxidation, while supplementation with the antioxidant CoQ10 decreased fragmentation and mitophagy Cornelius et al. While obesity and IEMs are distinct disorders, both conditions impinge on energy balance in WAT. Even though these disorders have very different manifestations, oxidative stress plays an important role in both and may be a therapeutic target. For example, CoQ10 is often given as a broad-spectrum treatment to individuals with IEMs, and while its effectiveness is debated, the anti-inflammatory effects may be beneficial in reducing oxidative stress and the pathogenesis of the disease Cornelius et al. Mitochondria represent control centers of many metabolic pathways. Interventions that enhance adipocyte mitochondrial function may also improve whole-body insulin sensitivity. Mitigation of mitochondrial ROS production and oxidative stress may be a possible therapeutic target in type 2 diabetes and IEMs because some mitochondrial-targeted antioxidants and other small molecule drugs improve metabolic profiles in mouse models Feillet-Coudray et al. Thiazolidinediones TZDs are PPARγ agonists used for treating type 2 diabetes Kelly et al. TZDs, such as rosiglitazone and pioglitazone, enhance insulin sensitivity by improving adipokine profiles Maeda et al. TZDs also promote insulin sensitivity by directing fatty acids to subcutaneous fat, rather than visceral fat. Subcutaneous fat expandability, even in the context of obesity and type 2 diabetes, correlates with insulin sensitivity in rodents and humans Ross et al. Numerous in vitro and in vivo studies demonstrate TZDs enhance mitochondrial biogenesis, content, function, and morphology. Rosiglitazone also induces cellular antioxidant enzymes responsible for the removal of ROS generated by increased mitochondrial activity in adipose tissue of diabetic rodents Rong et al. Taken together, TZDs impact WAT mitochondrial function in multiple ways that ultimately improve systemic fat metabolism and insulin sensitivity. Other therapeutic strategies include mitochondria-targeted scavengers Smith et al. However, these methods to enhance mitochondrial function display a narrow therapeutic range that limits safe use for obesity. Although the development of insulin resistance does not require impaired mitochondrial function Hancock et al. Aerobic exercise and caloric restriction disrupt this vicious loop, potentially by preventing accumulation of injured mitochondrial proteins with substantial improvement of insulin sensitivity. In insulin-resistant people, aerobic exercise stimulates both mitochondrial biogenesis and efficiency concurrent with an enhancement of insulin action Mul et al. Ultimately, exercise engages pathways that reduce ROS coupled with insulin sensitivity and improved mitochondrial function in WAT. Obesity is the result of excessive expansion of WAT depots due to a chronic imbalance between energy intake and expenditure. Many studies demonstrate that oxidative stress in fat cells links obesity and its comorbidities. The fact that WAT remains the sole organ for storing surfeit lipid renders the macromolecules in adipocytes particularly vulnerable to carbonylation and other modifications driven by oxidative stress. Prolonged oxidative stress negatively influences endocrine and homeostatic performance of WAT, including disruption of hormone secretion, elevation of serum lipids, inadequate cellular antioxidant defenses, and impaired mitochondrial function Figure 2. Metabolic challenges, such as persistent nutrient intake and sedentary behaviors that promote impaired glucose and lipid handling, also elevate mitochondrial ROS production to cause adipocyte dysfunction. Consequently, adipocytes cannot engage appropriate transcriptional and energetic responses to enable insulin sensitivity. Figure 2. Impact of oxidative stress on adipocyte function. Increased plasma glucose and free fatty acids contribute to increased oxidative stress by increasing the production of reactive oxygen species ROS and decreasing antioxidant concentrations. Increased oxidative stress occurs via enzymes in the cytoplasm, such as NADPH oxidase, and the mitochondria. The oxidative environment increases lipid storage resulting in hypertrophic adipocytes. Additionally, increased mitochondrial ROS mtROS alters the activity state of metabolic enzymes either directly or by changing the oxidative state of protein side-chains or by other post-translational modifications, including lipid peroxidation and protein carbonylation. Cumulatively, increased adipocyte oxidative stress decreases adipogenesis and secretion of adipokines, leading to unbalanced energy homeostasis, insulin resistance, and type 2 diabetes. The increasing prevalence of obesity suggests lifestyle intervention as the principal method to treat obesity is unlikely to succeed. Currently, all available anti-obesity medications act by limiting energy intake through appetite suppression or inhibition of intestinal lipid absorption. However, these medications are largely ineffective and often have adverse side effects. The central role of mitochondria in nutrient handling provides a logical entry point for improving metabolism in obesity. While approaches to understanding and intervening in oxidative damage evolve, exploration of mitochondria redox balance may enable development of dietary and small molecule therapies for obesity and its comorbidities. This work was funded by the American Diabetes Association IBS and NIH R01DK The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Acosta, M. Coenzyme Q biosynthesis in health and disease. Acta , — doi: PubMed Abstract CrossRef Full Text Google Scholar. Ahmed, M. Proteomic analysis of human adipose tissue after rosiglitazone treatment shows coordinated changes to promote glucose uptake. Obesity 18, 27— Akl, M. Perturbed adipose tissue hydrogen peroxide metabolism in centrally obese men: association with insulin resistance. PLoS One e Anderson, E. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. Armstrong, J. The redox regulation of intermediary metabolism by a superoxide-aconitase rheostat. Bioessays 26, — Barbosa, M. Hydrogen peroxide production regulates the mitochondrial function in insulin resistant muscle cells: effect of catalase overexpression. Bjelakovic, G. Antioxidant supplements to prevent mortality. JAMA , — Antioxidant supplements and mortality. Care 17, 40— Boden, G. Excessive caloric intake acutely causes oxidative stress, GLUT4 carbonylation, and insulin resistance in healthy men. Boden, M. Overexpression of manganese superoxide dismutase ameliorates high-fat diet-induced insulin resistance in rat skeletal muscle. Bogacka, I. Structural and functional consequences of mitochondrial biogenesis in human adipocytes in vitro. Bournat, J. Mitochondrial dysfunction in obesity. Diabetes Obes. Boyle, P. Effects of pioglitazone and rosiglitazone on blood lipid levels and glycemic control in patients with type 2 diabetes mellitus: a retrospective review of randomly selected medical records. Chappuis, B. Differential effect of pioglitazone PGZ and rosiglitazone RGZ on postprandial glucose and lipid metabolism in patients with type 2 diabetes mellitus: a prospective, randomized crossover study. Diabetes Metab. Chouchani, E. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature , — Mitochondrial reactive oxygen species and adipose tissue thermogenesis: bridging physiology and mechanisms. Cornelius, N. Secondary coenzyme Q10 deficiency and oxidative stress in cultured fibroblasts from patients with riboflavin responsive multiple Acyl-CoA dehydrogenation deficiency. Cellular consequences of oxidative stress in riboflavin responsive multiple acyl-CoA dehydrogenation deficiency patient fibroblasts. Curtis, J. Protein carbonylation and metabolic control systems. Trends Endocrinol. Dasuri, K. Role of physiological levels of 4-hydroxynonenal on adipocyte biology: implications for obesity and metabolic syndrome. Free Radic. Davies, M. Protein oxidation and peroxidation. De la Mata, M. Recovery of MERRF fibroblasts and cybrids pathophysiology by coenzyme Q Neurotherapeutics 9, — Deeg, M. Pioglitazone and rosiglitazone have different effects on serum lipoprotein particle concentrations and sizes in patients with type 2 diabetes and dyslipidemia. Diabetes Care 30, — Demozay, D. FALDH reverses the deleterious action of oxidative stress induced by lipid peroxidation product 4-hydroxynonenal on insulin signaling in 3T3-L1 adipocytes. Elrayess, M. Escribano-Lopez, I. The mitochondrial antioxidant SS increases SIRT1 levels and ameliorates inflammation, oxidative stress and leukocyte-endothelium interactions in type 2 diabetes. Esterbauer, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Fazakerley, D. Mitochondrial oxidative stress causes insulin resistance without disrupting oxidative phosphorylation. Feillet-Coudray, C. The mitochondrial-targeted antioxidant MitoQ ameliorates metabolic syndrome features in obesogenic diet-fed rats better than Apocynin or Allopurinol. Fouret, G. The mitochondrial-targeted antioxidant, MitoQ, increases liver mitochondrial cardiolipin content in obesogenic diet-fed rats. Frohnert, B. Protein carbonylation, mitochondrial dysfunction, and insulin resistance. Increased adipose protein carbonylation in human obesity. Obesity 19, — Furukawa, S. Increased oxidative stress in obesity and its impact on metabolic syndrome. PubMed Abstract Google Scholar. Fusco, D. Effects of antioxidant supplementation on the aging process. Aging 2, — Gardner, P. Superoxide radical and iron modulate aconitase activity in mammalian cells. Goldberg, R. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care 28, — Goldgof, M. The chemical uncoupler 2,4-dinitrophenol DNP protects against diet-induced obesity and improves energy homeostasis in mice at thermoneutrality. Grimsrud, P. Carbonylation of adipose proteins in obesity and insulin resistance: identification of adipocyte fatty acid-binding protein as a cellular target of 4-hydroxynonenal. Proteomics 6, — Oxidative stress and covalent modification of protein with bioactive aldehydes. Han, Y. Adipocyte-specific deletion of manganese superoxide dismutase protects from diet-induced obesity through increased mitochondrial uncoupling and biogenesis. Hancock, C. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Hauck, A. Adipose oxidative stress and protein carbonylation. Obesity-induced protein carbonylation in murine adipose tissue regulates the DNA-binding domain of nuclear zinc finger proteins. Hausladen, A. Superoxide and peroxynitrite inactivate aconitases, but nitric oxide does not. Holley, A. Manganese superoxide dismutase: guardian of the powerhouse. Holloszy, J. Huh, J. Peroxiredoxin 3 is a key molecule regulating adipocyte oxidative stress, mitochondrial biogenesis, and adipokine expression. Hurd, T. Inactivation of pyruvate dehydrogenase kinase 2 by mitochondrial reactive oxygen species. Kelly, I. Effects of a thiazolidinedione compound on body fat and fat distribution of patients with type 2 diabetes. Diabetes Care 22, — Khan, M. A prospective, randomized comparison of the metabolic effects of pioglitazone or rosiglitazone in patients with type 2 diabetes who were previously treated with troglitazone. Diabetes Care 25, — Kim, J. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. King, A. A comparison in a clinical setting of the efficacy and side effects of three thiazolidinediones. Diabetes Care 23, Kowaltowski, A. Mitochondrial damage induced by conditions of oxidative stress. Lee, H. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. Mitochondrial-targeted catalase protects against high-fat diet-induced muscle insulin resistance by decreasing intramuscular lipid accumulation. Liesa, M. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. Long, E. High-fat diet induces changes in adipose tissue transoxononenal and transhydroxynonenal levels in a depot-specific manner. Lu, R. Mitochondrial development and the influence of its dysfunction during rat adipocyte differentiation. Lushchak, O. Aconitase post-translational modification as a key in linkage between Krebs cycle, iron homeostasis, redox signaling, and metabolism of reactive oxygen species. Redox Rep. Maeda, N. PPARgamma ligands increase expression and plasma concentrations of adiponectin, an adipose-derived protein. |
| Human Verification | Ginseng for cognitive function etomoxir and perhexiline CPT1 inhibitorsit is not known Augmentee SSO metablism hepatotoxicity. We show here Augmented fat metabolism efficiency in response to muscle injury, ASCs within ScAT overexpress podoplanin, which is a ligand for the platelet receptor CLEC-2 for C-type lectin-like receptor 2 Powered by University of Utah. Horm Metab Res. J Biol Chem. |
| Frontiers | Loss of Metabolic Flexibility in the Failing Heart | Because FAs are chemically more reduced molecules than carbohydrates, FAs are theoretically able to produce more energy when completely oxidised than an equivalent carbohydrate molecule. In other words, the complete oxidation of six-carbon glucose consumes six oxygen molecules and produces six carbon dioxide molecules accompanied by the synthesis of 36 ATP molecules. On the other hand, the complete oxidation of six-carbon hexanoic acid consumes eight oxygen molecules and produces six carbon dioxide molecules for 44 ATP molecules. Therefore, a switch to the oxidation of FAs as the major energy substrate should result in less efficient ATP production and an increase in whole-body energy expenditure that could lead to a loss of fat mass if energy intake remains constant Clapham a , b , Leverve et al. Pathways of substrate metabolism in muscle. Oxidation pathways of glucose, FAs and amino acids converge at the level of acetyl CoA. This proton motive force is dissipated by ATP synthesis and by proton leak via the adenine nucleotide transporter ANT and activated uncoupling proteins UCPx. Demand for ATP and proton leak are greater determinants of oxygen consumption and heat production than the substrate being oxidised. Citation: Journal of Endocrinology , 2; Indirect calorimetry is often used in human and animal studies to determine total energy expenditure indirectly by the measurement of oxygen consumption and carbon dioxide production , and the measurement of oxygen consumption and carbon dioxide production can also be used to calculate the relative use of glucose and FAs to support that energy expenditure respiratory exchange ratio, RER , assuming that any contribution of protein oxidation is relatively small and constant Ferrannini , Arch et al. However, in practice, it is unlikely that such theoretical calculations can be applied to the regulation of energy balance with any certainty. For instance, rarely does the measured RER shift from complete glucose oxidation 1. counter-ion transport and uncoupling protein activity Mazat et al. The concepts of efficiency and plasticity in the coupling of substrate oxidation to energy conservation ATP synthesis have been expanded on in several authoritative review articles Harper et al. In reality, coupling efficiency can vary significantly depending on changes in proton leak or ATP demand, but in cell systems at least, changes in substrate oxidation do not appear to influence the relationship between oxygen consumption and ATP synthesis Brand et al. The above discussion clearly leads to the conclusion that the cost of generating mitochondrial ATP in terms of ETC activity and oxygen consumption can vary significantly and is not affected to any large extent by the substrate being oxidised to provide the reducing equivalents for electron transport. Despite this, it is not uncommon to read about studies in whole animal systems particularly genetically modified mice where differences in fat mass are often mechanistically related to changes in the mRNA levels of FA metabolism genes in a variety of tissues without appropriate consideration of the contribution of these tissues to whole-body energy expenditure Abu-Elheiga et al. For example, expression of oxygen consumption or heat production on a kilogram body weight basis can be misleading if animals have significantly different amounts of fat tissue, because the metabolic rate of fat per gram is much lower in tissues such as muscle and liver Frayn et al. Similarly, the difference in daily food intake needed to contribute to a significant gain of body fat over several weeks in mice can be so small as to be undetectable unless large numbers of mice — are used for the comparison Tschop et al. Changes in the body weight and body fat of groups of adult mice with different genotypes on different diets should reflect cumulative differences in energy intake and energy expenditure. However, any differences might not be easily detected if animals are assessed for food intake and energy expenditure individually in indirect calorimetry systems, away from their home cage and communal environment for only a 24—h period of the several weeks over which body weight and fat mass have been monitored. AMP-activated protein kinase AMPK is recognised as a master regulator of energy metabolism, particularly in times of energy stress such as exercise, hypoxia and starvation Hardie et al. The activation of AMPK has been shown to acutely increase FA and glucose uptake and metabolism in a variety of experimental situations including in vitro and in vivo experiments in muscle Iglesias et al. The long-term effects of AMPK activation in muscle lead to the activation of gene transcription pathways that increase mitochondrial biogenesis and proteins of oxidative metabolism Winder et al. The acute regulation of FA oxidation by AMPK is largely through the phosphorylation and inactivation of the enzyme acetyl CoA carboxylase 2 ACC2. The pharmacological activation of AMPK has been shown to produce changes in muscle metabolic pathway capacity similar to those produced by exercise training O'Neill et al. A series of studies employing genetic deletion of Acc2 Acacb have reported reduced fat depots in association with increased FA oxidation in isolated muscle Abu-Elheiga et al. These results suggest that the inhibition of ACC2 by the activation of AMPK or development of ACC2 inhibitors might promote FA oxidation and produce fat loss. Subsequent studies using independently generated Acc2 -knockout mice did not reproduce these effects, reporting that although these mice exhibited increased FA oxidation at the whole-body and isolated muscle level, there was no measurable difference in energy expenditure, fat mass or food intake Hoehn et al. However, there was an increased glycogen content in muscle, an effect of AMPK activation noted previously Winder et al. Another study using independently generated genetically manipulated mice has reported no difference in body weight, food intake or fat mass in global or muscle-specific Acc2 gene-deleted mice Olson et al. Therefore, it would appear that apart from theoretical calculations suggesting that increasing fat oxidation will drive increased energy expenditure, there is little experimental evidence to support the idea that energy expenditure can be increased simply by increasing substrate availability or by switching to oxidise FAs. From an energy metabolism point of view, the flow of different substrates to tissues for oxidation or storage is largely under the control of the circulating hormone insulin. After a meal, direct stimulation of the β-cells of the islets of Langerhans of the pancreas by nutrients glucose, FAs and amino acids increases insulin release into the circulation. Certain gut hormones GLP1 and GP can also augment insulin secretion, as can neural signals from the brain Thorens Insulin has many stimulatory and inhibitory actions in different tissues mediated by a complex intracellular signalling pathway, but for the purpose of this discussion, the actions of insulin to stimulate glucose uptake and metabolism in muscle and regulate FA metabolism will be a major focus. The failure of insulin to appropriately regulate glucose and FA metabolism is termed insulin resistance, and this condition is most frequently observed in the muscle and liver of overweight or obese individuals Eckardt et al. Insulin resistance is considered a significant predisposing factor for the development of type 2 diabetes T2D and therefore there is considerable research effort put into determining the mechanistic relationship between excess lipid accumulation obesity and insulin resistance, particularly in muscle. Studies from over 20 years ago first showed that triglyceride accumulation in the muscle of high-fat diet-fed rats coincided with insulin resistance Storlien et al. Since then, the relationship between muscle lipid accumulation and insulin resistance has also been established in humans, and many mechanisms have been put forward to explain how lipid accumulation could generate insulin resistance Bosma et al. Over the last decade, the major challenge has been determining whether these proposed mechanisms are universal or specific to the model of lipid-induced insulin resistance being studied. It is also possible that different mechanisms are important at different times during the development of insulin resistance and that some proposed mechanisms depend on the experimental methods used to assess insulin action. All discussions of the relationship between increased fat metabolism and insulin action are dependent on the methodology used to assess insulin resistance and the assumptions associated with different methodologies. As has been mentioned previously, nearly all investigations of lipid-induced insulin resistance in rodent models utilise high-fat diet feeding to increase adiposity, but the methods of assessing insulin action can be quite varied and rely on glucose tolerance tests or insulin tolerance tests and less frequently because of the technical difficulty on hyperinsulinaemic—euglycaemic clamps. Various technical considerations of glucose and insulin tolerance tests must be considered when discussing the metabolic implications of these tests for muscle insulin action. The timing and route of administration of glucose and duration of fast before glucose administration influence the results of glucose tolerance tests Andrikopoulos et al. Insulin tolerance tests were devised largely to assess the effectiveness of counter-regulatory mechanisms in response to insulin-induced hypoglycaemia and therefore the utility of these tests to assess peripheral insulin action is debatable. Neither glucose tolerance nor insulin tolerance tests give specific data regarding insulin effectiveness in muscle, although several methodological variations have used concurrent injection of radioactive tracers to assess glucose clearance into muscle during a glucose tolerance or insulin tolerance test Crosson et al. The hyperinsulinaemic—euglycaemic clamp with glucose tracer administration gives the most reproducible assessment of muscle glucose clearance in response to constant insulin stimulation and constant glucose availability Ayala et al. This technique relies on plasma insulin levels not insulin infusion rates during the comparison of the clamp being matched between the groups. In many studies, plasma insulin levels during the clamp are not reported, making the assessment of muscle insulin action difficult Chapman et al. In vitro assessment of insulin effectiveness in isolated soleus or extensor digitorum longus muscle is also often used to demonstrate the effects of FA exposure Thompson et al. reliance on diffusion and not on perfusion. While all the above methods can give useful information about the effects of muscle lipid accumulation on insulin action, this information can be specific for the test employed. Even the data obtained from hyperinsulinaemic—euglycaemic clamp studies describe fluxes measured after at least an hour of exposure to constant insulin stimulation and constant glucose availability, a situation that is unlikely to ever exist in the normal h feeding—fasting cycle. Therefore, it would seem important to consider the method used to demonstrate a difference in insulin action with lipid accumulation, when assessing the relevance of various mechanisms to reduced glucose metabolism in muscle when no restrictive experimental conditions e. in vitro assessment, constant infusion and i. As has been mentioned above, the association between intramyocellular triglyceride IMTG content and insulin resistance is now well established in animals and obese humans, and most studies investigating the mechanisms of insulin resistance in muscle use high-fat diet rodent models. It has also become standard practice in assessing the phenotype of genetically manipulated mice to place them on high-fat diets to investigate whether there is any impact favourable or detrimental of gene manipulation on glucose and energy homoeostasis. There is a reasonable assumption that, independent of genetic background in animals or humans, overconsumption of energy-dense diets plays a major role in the accumulation of fats and development of metabolic derangements in muscle. In humans, overconsumption of energy-dense diets for a few weeks is enough to increase fat mass and have detrimental effects on whole-body insulin action Samocha-Bonet et al. In mice, high-fat feeding for as little as a few days can impair glucose tolerance Turner et al. Studies that improved insulin sensitivity by low-calorie diets in patients with T2D were accompanied by a reduction in IMTG content Jazet et al. Insulin resistance associated with ageing Nakagawa et al. Current opinion is reasonably clear on the fact that IMTG is a useful marker of the level of cytosolic lipid accumulation, but it is more likely that active lipid metabolites such as LCACoAs, DAGs and ceramides or intermediates of FA oxidation pathways interfere with insulin action via a variety of potential mechanisms Fig. These mechanisms are largely based on the idea that insulin resistance in muscle is the result of reduced transduction of the insulin signal through the phosphorylation cascade leading to the translocation of the glucose transporter GLUT4 to the sarcolemmal membrane Stockli et al. Since that time, research into the molecular mechanism of FA-induced insulin resistance in muscle has mainly focused on linking excess FAs to defects in the insulin signalling pathways that regulate glucose uptake. However, there are some established and some more speculative mechanisms that also link increased FA metabolism with reduced insulin action, and these are discussed in the subsequent sections. Proposed mechanisms for the build-up of bioactive lipid species and how they interfere with insulin action in muscle to produce insulin resistance. DAG can activate lipid-sensitive kinases to serine phosphorylate and reduce tyrosine phosphorylation of IRS1. Ceramide can inhibit Akt phosphorylation and reduce transduction through the insulin signalling pathway. Circulating cytokines or FAs themselves are reported to activate inflammatory pathway serine kinases that interfere with insulin signalling. Reduced or dysregulated FA oxidation in mitochondria could create a build-up of bioactive lipids and generate reactive oxygen species ROS that also activate kinases that interfere with insulin signalling. IMTGs are considered to be relatively benign with regard to insulin resistance Goodpaster et al. However, despite the general consensus that IMTGs are metabolically inert, it is possible that the expanded IMTG pool generates intermediates of lipid metabolism that are more likely to play a mechanistic role in the development of muscle insulin resistance. In this respect, the bioactive lipid metabolites DAG and ceramide are leading candidates. The levels of both DAG Turinsky et al. While less is known about the role of these lipids in humans, it has been reported that acute lipid-induced insulin resistance is associated with muscle DAG accumulation Itani et al. Furthermore, interventions that enhance insulin action, such as exercise training, cause reductions in muscle DAG and ceramide content Bruce et al. Specifically, DAG accumulation is thought to impair insulin action via the activation of novel protein kinase C PKC isoforms, which subsequently inhibits insulin signal transduction to glucose transport via serine phosphorylation of insulin receptor substrate 1 IRS1; Yu et al. Ceramide has been reported to cause insulin resistance by impairing insulin signalling at the level of Akt Schmitz-Peiffer et al. In addition, ceramide is a potent activator of inflammatory molecules, including c-Jun N-terminal kinase JNK; Westwick et al. However, while inflammation has been proposed as a critical factor causing insulin resistance, studies carried out by our group and other groups suggest that inflammation is not involved in the initiation of lipid-induced insulin resistance, but may be more important in the exacerbation and maintenance of insulin resistance once obesity is established Lee et al. Although there is mounting evidence supporting a role for DAG and ceramide in the regulation of insulin sensitivity, it is important to highlight that the accumulation of these lipids is not always associated with insulin resistance. In fact, a recent study has found that total DAG content is actually elevated in the muscle of highly insulin-sensitive endurance-trained athletes compared with the skeletal muscle of obese individuals Amati et al. Furthermore, a positive correlation between total muscle ceramide content and insulin sensitivity has been reported Skovbro et al. These data suggest a more complex role for DAG and ceramide in the regulation of insulin action Amati et al. While the bioactive lipid hypothesis has gained strong support, an alternative concept linking the accumulation of intermediates of mitochondrial FA oxidation with muscle insulin resistance has gained attention Koves et al. This model proposes that lipid oversupply drives an increase in mitochondrial β-oxidation that exceeds the capacity of the Krebs cycle, leading to the accumulation of by-products of FA oxidation Koves et al. This is supported by studies demonstrating an increase in incomplete FA oxidation and an accompanying increase in intramuscular acylcarnitine levels in obese rodents Koves et al. While data in humans are currently limited, there is evidence that acylcarnitine does accumulate in the muscle of humans in response to a high-fat diet Putman et al. However, it is not clear whether acylcarnitine plays a direct role in the modulation of skeletal muscle insulin sensitivity by disrupting signalling processes or whether it simply reflects a state of mitochondrial stress. Unravelling the role of acylcarnitine in muscle insulin sensitivity will no doubt be a focus of future research. Another prominent theory on the aetiology of insulin resistance implicates abnormalities in mitochondrial function as a major causative factor leading to reductions in insulin sensitivity. More specifically, defects in mitochondrial metabolism have been suggested to lead to inadequate substrate oxidation, precipitating a build-up of intracellular lipid metabolites, impaired insulin signalling and the subsequent development of insulin resistance Lowell and Shulman , Kim et al. The initial studies that set the platform for this theory in the late s showed that there was reduced mitochondrial enzyme activity and decreased fat oxidation in the skeletal muscle of obese, insulin-resistant subjects and in individuals with T2D Kelley et al. Kelley et al. In the following year, two prominent microarray studies were published, describing a coordinated down-regulation of genes involved in mitochondrial biogenesis and oxidative phosphorylation in subjects with T2D and, importantly, also in non-diabetic individuals with a family history of T2D Mootha et al. In the ensuing decade since the publication of these landmark studies, many groups have reported defects in different mitochondrial parameters in the skeletal muscle of a range of different insulin-resistant populations obese, T2D and PCOS. Functional studies in muscle biopsy samples or in vivo using magnetic resonance spectroscopy have also reported decreases in mitochondrial oxidative capacity in insulin-resistant individuals Petersen et al. Collectively, all these studies suggest that at some level, mitochondria in insulin-resistant individuals are not as effective at burning fuel substrates in muscle and this compromises insulin action. Despite the large body of evidence described above, this area is controversial, as many studies report a dissociation between insulin resistance and mitochondrial dysfunction. For example, providing rodents with excess fat in their diet leads to an enhancement of mitochondrial oxidative capacity in muscle while at the same time inducing insulin resistance Turner et al. Several lines of mice with genetic manipulations that cause compromised mitochondrial function in muscle do not exhibit insulin resistance Vianna et al. Conversely, two separate lines of muscle-specific Pgc1 α Ppargc1a transgenic mice displayed a significant enhancement in the markers of mitochondrial content and yet were insulin resistant due to excessive FA delivery and reduced GLUT4 SLC2A4 expression in muscle Miura et al. A growing number of studies in humans have also reported intact mitochondrial function in various insulin-resistant populations De Feyter et al. Collectively, these studies suggest that mitochondrial dysfunction in muscle is not an obligatory factor required for the accumulation of intramuscular lipids and the development of insulin resistance. normal free-living conditions Hancock et al. In addition to their role as major sites for energy transduction, mitochondria are also known to be a major source of reactive oxygen species ROS , which are produced as a by-product of normal metabolic reactions Andreyev et al. ROS have the capacity to damage macromolecules, and when the production of these reactive species is in excess of the antioxidant defences, a state of oxidative stress results. FA catabolism is known to promote mitochondrial ROS production St-Pierre et al. Importantly, many studies have shown that insulin action is improved when mitochondrial ROS production is attenuated Houstis et al. Before the elucidation of the insulin signalling pathway and recognition of the complex processes involved in the translocation of GLUT4 from intracellular vesicles to sarcolemmal membrane, there was a large amount of experimental data pointing to significant FA regulation of glucose metabolism at the level of PDH Randle et al. If humans, animals or in vitro preparations of muscle are exposed to an increased availability of FAs in the presence of glucose, the oxidation of FAs increases and the oxidation and uptake of glucose decrease Boden et al. On the other hand, reduction of the availability of FAs by inhibiting lipolysis Vaag et al. Although the initial observations of Randle and colleagues on the reciprocal relationship between glucose and FA metabolism were made 50 years ago, the idea that increasing or reducing FA availability will reciprocally affect glucose utilisation is no less valid today. Therefore, in the context FA-induced insulin resistance, a role for substrate competition and regulation at the level of PDH should not be overlooked. As outlined in other sections of this review, the current dogma suggests that the major mechanisms for FA-induced insulin resistance in muscle involve active lipid species interfering with insulin signalling via the activation of various serine kinases Fig. The canonical insulin signalling cascade comprises scaffolding proteins e. IRS1 and enzymes e. Mitochondrial insufficiency and ROS are also thought to feedback and impinge on the efficiency of insulin signalling via the activation of regulatory kinases. While there are many studies showing clear differences in the phosphorylation status of various insulin signalling proteins after insulin stimulation in control and FA-exposed or obese or high-fat diet-fed muscle, these changes are not always consistent. There are a number of studies reporting that insulin-stimulated Akt activation is in fact not impaired in the muscle of obese individuals with insulin resistance, of glucose-intolerant first-degree relatives of patients with T2D and of patients with T2D Kim et al. This dissociation between measured changes in insulin-stimulated glucose flux and insulin effects on signalling proteins has a number of implications. First, it might highlight the technical difficulties of obtaining reliable, quantitative data on protein modification using the essentially non-quantitative technique of immunoblotting. The ability to detect differences with this methodology can also depend on the affinity of individual antibodies, and the amount of phosphorylation does not necessarily correlate linearly with the activity of the signalling protein. The introduction of mass spectrometry techniques to analyse changes in global protein phosphorylation in response to insulin, as has been applied in adipocytes Humphrey et al. Another possibility is that phosphorylation is not the only post-translational modification of proteins involved in the generation of lipid-induced insulin resistance. Recently, the emergence of nitrosative modifications White et al. Another area of research that is increasingly realised to have a significant impact on metabolic disease is circadian biology. The suprachiasmatic nucleus in the brain is considered to be the master regulator of circadian behaviour because of its ability to coordinate inputs from the environment light, food, exercise and temperature , but it is now clear that every tissue has the molecular components that comprise the clock, raising the possibility that circadian processes in tissues could be regulated directly by some inputs. Some mouse models with genetic manipulations of core clock genes have altered circadian rhythms and are more prone to developing obesity Turek et al. If there is an underlying rhythm to metabolism in muscle driven by the molecular clock Lefta et al. In fact, a recent report has suggested that the time of day can have a significant effect on the data obtained from euglycaemic-hyperinsulinaemic clamps in mice Shi et al. The correlation between increased FA availability and reduced insulin-stimulated glucose metabolism is well established. Despite this clear relationship, to date, there has been no unifying mechanism that explains lipid-induced reductions in insulin action under all circumstances. However, there are an increasing number of experimental situations where reduced effects of insulin in muscle have been observed without significant changes in the phosphorylation of signalling proteins or where differences in phosphorylation are only observed with stimulation by supraphysiological insulin concentrations. This suggests that other control mechanisms or other forms of protein modification may predominate depending on the exact experimental conditions used to examine insulin resistance e. bolus insulin injections, hyperinsulinaemic clamps and glucose or lipid infusion. Figure 3 summarises some of the key control points other than insulin signalling for GLUT4 translocation that could alter the balance between glucose and FA metabolism and affect insulin-stimulated glucose disposal. For example, utilisation of glucose and FAs is dependent on their availability in the circulation and delivery to the muscle tissue, and changes in microvasculature occur with obesity and contribute to muscle insulin resistance St-Pierre et al. Other work Furler et al. The phosphorylation of glucose by hexokinase and the pathway for conversion of glucosephosphate to glycogen are subject to regulation by glucosephosphate and glycogen respectively, and decreased glucose phosphorylation and glycogen synthesis will affect glucose uptake Fueger et al. Another well-documented node regulating the metabolism of glucose is centred on the activity of PDH. The activity of this enzyme complex is inhibited by phosphorylation via PDH kinase 4 PDK4. Interestingly, the amount of PDK4 in muscle is significantly increased in high-fat diet-fed, insulin-resistant animals and PDK4 is activated by acetyl CoA, providing evidence that this regulatory node could significantly affect glucose metabolism in muscle as hypothesised by Newsholme and Randle many years ago Randle et al. Nodes of control of glucose metabolism other than insulin-stimulated translocation of GLUT4 that could be influenced by the excess availability of FAs. Utilisation of glucose and FAs is dependent on their availability in the circulation and delivery to the muscle tissue. The phosphorylation of glucose and conversion to glycogen are regulated by substrate availability and GP concentration. PDH is a critical regulator balancing glucose use and FA oxidation to support energy requirements. The regulation of FA sequestration in, or release from, muscle fat droplets can control the level of bioactive lipid species. The regulation of FA metabolism at the AMPK—ACC2—malonyl CoA—CPT1 axis also has a significant impact on the balance between FA and glucose metabolism. There are a number of newly recognised post-translational modifications that can occur on key metabolic or signalling proteins and would be expected to be influenced by changes in the availability and metabolism of FAs. FA metabolism in muscle can also be regulated at the membrane by transporter proteins such as CD36 , and at activation to acyl CoA by acyl CoA synthase Glatz et al. The partitioning of FAs towards triglyceride storage or mitochondrial oxidation may depend on the activity of key enzymes such as glycerol phosphate acyltransferase and adipose triglyceride lipase Greenberg et al. The entry of long-chain FAs into the mitochondria for oxidation is thought to be largely regulated by the activity of CPT1. The activity of CPT1 is modulated allosterically by malonyl CoA, and numerous studies, including our recently published papers using genetic and pharmacological interventions Bruce et al. Depending on the experimental design used, acutely increasing fatty oxidation in muscle can decrease glucose utilisation Hoehn et al. Interestingly, acute blockade of FA oxidation increases insulin-stimulated glucose uptake Oakes et al. These differences in acute and chronic responses when substrate metabolism is manipulated may be reconciled by considering the fact that energy metabolism is not constant in animals and humans, but has a substantial diurnal variation that is highly relevant to designing appropriate experiments to investigate lipid-induced insulin resistance. In conclusion, it may be unrealistic to expect that a unifying mechanism may explain all situations where there is reduced glucose metabolism in muscle in response to insulin, as multiple factors may contribute to the establishment and long-term maintenance of insulin resistance in this tissue. With the emergence of powerful techniques for determining global changes in gene expression, protein modifications and metabolite profiles, it will hopefully become possible to gain a more comprehensive idea of the factors and pathways that may contribute to the aetiology of lipid-induced insulin resistance in muscle. The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the review. The work carried out in the laboratories of the authors is supported by Program and Project grant funding from the National Health and Medical Research Council of Australia NHMRC , the Australian Research Council ARC and the Diabetes Australia Research Trust. NT is supported by an ARC Future Fellowship. G J C and E W K hold research fellowships from the NHMRC and C R B has received a career development award from the NHMRC. Science — PNAS — International Journal of Obesity 33 — American Journal of Physiology. Endocrinology and Metabolism E — E Diabetes 60 — Journal of Clinical Investigation — Biochemistry 70 — International Journal of Obesity 30 — Diabetes 55 — Bangsbo J Muscle oxygen uptake in humans at onset of and during intense exercise. Acta Physiologica Scandinavica — Bass J Circadian topology of metabolism. Nature — Diabetes 56 — Diabetologia 55 — Journal of Clinical Investigation 93 — Annual Review of Nutrition 33 — Progress in Lipid Research 51 36 — Diabetologia 50 — Cell Metabolism 12 — Biochemical Journal — International Journal of Obesity 37 — Diabetes 58 — Endocrinology 65 — Endocrinology and Metabolism E99 — E Diabetes 50 12 — Diabetes 59 — PLoS ONE 5 e Clapham JC a Fat oxidation in obesity: druggable or risky enterprise? IDrugs: the Investigational Drugs Journal 7 — Clapham JC b Treating obesity: pharmacology of energy expenditure. Current Drug Targets 5 — Molecular Endocrinology 21 — Trends in Endocrinology and Metabolism 23 — Experimental Physiology 92 — FASEB Journal 27 — EMBO Journal 23 — One carbonylated protein of importance was GLUT4, whose carbonylation likely impairs insulin-stimulated glucose uptake. Of note, systemic oxidative stress and insulin resistance did not coincide with inflammatory cytokines in plasma nor ER stress in WAT. These findings provide a causal link between oxidative stress and insulin resistance in humans. Mitochondrial metabolism is often altered in inherited diseases, such as inborn errors of metabolism IEMs that impinge upon ROS generation. Inhibition of OXPHOS increases ROS generation due to a backlog of electrons in the various complexes, resulting in electron leak, ROS generation, and production of H 2 O 2. In IEMs affecting the ETC or other pathways of ATP generation, increased oxidative stress is often observed, while the exact mechanisms for increased ROS production are unknown. It is hypothesized that mutations affecting the formation of the protein complexes in the ETC or mutations that modify their assembly increase ROS generation by facilitating electron leak Olsen et al. Additionally, accumulation of toxic intermediates, often observed in IEMs, can increase the ROS generation by further decreasing OXPHOS activity, as in the case of medium-chain acyl-CoA dehydrogenase MCAD deficiency. MCAD deficiency reflects the accumulation of medium-chain fatty acid derivatives, including cisdecenoic acid, octanoate, and decanoate, with these metabolites altering levels of antioxidants and increasing markers of oxidative stress Schuck et al. Intriguingly, IEMs display metabolic reprograming with a switch to glycolysis for both ATP production and muted ROS generation Olsen et al. Specifically, in myoclonic epilepsy with ragged red fibers MERRF , increased intracellular H 2 O 2 levels correspond with increased AMPK phosphorylation and expression of GLUT1, hexokinase II, and lactate dehydrogenase. These results, as well as increased lactic acid production, all point to increased glycolysis De la Mata et al. In multiple acyl-CoA dehydrogenase deficiency MADD , mutations in ETFa , ETFb , or ETFDH , lead to decreased ATP production with an accumulation of organic acids, including glutaric acid as well as acyl-carnitines. A subset of these patients is riboflavin responsive RR-MADD with high dose riboflavin alleviating some symptoms. Similar to MERRF, many RR-MADD patients exhibit increased oxidative stress Cornelius et al. This defect may be due to defective electron transfer and increased electron leak from the misfolded ETFDH protein and decreased binding of CoQ10 Cornelius et al. Treatment with CoQ10, but not riboflavin, decreased ROS levels Cornelius et al. Analysis of mitochondrial function from RR-MADD fibroblasts showed increased mitochondrial fragmentation and reduced β-oxidation, while supplementation with the antioxidant CoQ10 decreased fragmentation and mitophagy Cornelius et al. While obesity and IEMs are distinct disorders, both conditions impinge on energy balance in WAT. Even though these disorders have very different manifestations, oxidative stress plays an important role in both and may be a therapeutic target. For example, CoQ10 is often given as a broad-spectrum treatment to individuals with IEMs, and while its effectiveness is debated, the anti-inflammatory effects may be beneficial in reducing oxidative stress and the pathogenesis of the disease Cornelius et al. Mitochondria represent control centers of many metabolic pathways. Interventions that enhance adipocyte mitochondrial function may also improve whole-body insulin sensitivity. Mitigation of mitochondrial ROS production and oxidative stress may be a possible therapeutic target in type 2 diabetes and IEMs because some mitochondrial-targeted antioxidants and other small molecule drugs improve metabolic profiles in mouse models Feillet-Coudray et al. Thiazolidinediones TZDs are PPARγ agonists used for treating type 2 diabetes Kelly et al. TZDs, such as rosiglitazone and pioglitazone, enhance insulin sensitivity by improving adipokine profiles Maeda et al. TZDs also promote insulin sensitivity by directing fatty acids to subcutaneous fat, rather than visceral fat. Subcutaneous fat expandability, even in the context of obesity and type 2 diabetes, correlates with insulin sensitivity in rodents and humans Ross et al. Numerous in vitro and in vivo studies demonstrate TZDs enhance mitochondrial biogenesis, content, function, and morphology. Rosiglitazone also induces cellular antioxidant enzymes responsible for the removal of ROS generated by increased mitochondrial activity in adipose tissue of diabetic rodents Rong et al. Taken together, TZDs impact WAT mitochondrial function in multiple ways that ultimately improve systemic fat metabolism and insulin sensitivity. Other therapeutic strategies include mitochondria-targeted scavengers Smith et al. However, these methods to enhance mitochondrial function display a narrow therapeutic range that limits safe use for obesity. Although the development of insulin resistance does not require impaired mitochondrial function Hancock et al. Aerobic exercise and caloric restriction disrupt this vicious loop, potentially by preventing accumulation of injured mitochondrial proteins with substantial improvement of insulin sensitivity. In insulin-resistant people, aerobic exercise stimulates both mitochondrial biogenesis and efficiency concurrent with an enhancement of insulin action Mul et al. Ultimately, exercise engages pathways that reduce ROS coupled with insulin sensitivity and improved mitochondrial function in WAT. Obesity is the result of excessive expansion of WAT depots due to a chronic imbalance between energy intake and expenditure. Many studies demonstrate that oxidative stress in fat cells links obesity and its comorbidities. The fact that WAT remains the sole organ for storing surfeit lipid renders the macromolecules in adipocytes particularly vulnerable to carbonylation and other modifications driven by oxidative stress. Prolonged oxidative stress negatively influences endocrine and homeostatic performance of WAT, including disruption of hormone secretion, elevation of serum lipids, inadequate cellular antioxidant defenses, and impaired mitochondrial function Figure 2. Metabolic challenges, such as persistent nutrient intake and sedentary behaviors that promote impaired glucose and lipid handling, also elevate mitochondrial ROS production to cause adipocyte dysfunction. Consequently, adipocytes cannot engage appropriate transcriptional and energetic responses to enable insulin sensitivity. Figure 2. Impact of oxidative stress on adipocyte function. Increased plasma glucose and free fatty acids contribute to increased oxidative stress by increasing the production of reactive oxygen species ROS and decreasing antioxidant concentrations. Increased oxidative stress occurs via enzymes in the cytoplasm, such as NADPH oxidase, and the mitochondria. The oxidative environment increases lipid storage resulting in hypertrophic adipocytes. Additionally, increased mitochondrial ROS mtROS alters the activity state of metabolic enzymes either directly or by changing the oxidative state of protein side-chains or by other post-translational modifications, including lipid peroxidation and protein carbonylation. Cumulatively, increased adipocyte oxidative stress decreases adipogenesis and secretion of adipokines, leading to unbalanced energy homeostasis, insulin resistance, and type 2 diabetes. The increasing prevalence of obesity suggests lifestyle intervention as the principal method to treat obesity is unlikely to succeed. Currently, all available anti-obesity medications act by limiting energy intake through appetite suppression or inhibition of intestinal lipid absorption. However, these medications are largely ineffective and often have adverse side effects. The central role of mitochondria in nutrient handling provides a logical entry point for improving metabolism in obesity. While approaches to understanding and intervening in oxidative damage evolve, exploration of mitochondria redox balance may enable development of dietary and small molecule therapies for obesity and its comorbidities. This work was funded by the American Diabetes Association IBS and NIH R01DK The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Acosta, M. Coenzyme Q biosynthesis in health and disease. Acta , — doi: PubMed Abstract CrossRef Full Text Google Scholar. Ahmed, M. Proteomic analysis of human adipose tissue after rosiglitazone treatment shows coordinated changes to promote glucose uptake. Obesity 18, 27— Akl, M. Perturbed adipose tissue hydrogen peroxide metabolism in centrally obese men: association with insulin resistance. PLoS One e Anderson, E. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. Armstrong, J. The redox regulation of intermediary metabolism by a superoxide-aconitase rheostat. Bioessays 26, — Barbosa, M. Hydrogen peroxide production regulates the mitochondrial function in insulin resistant muscle cells: effect of catalase overexpression. Bjelakovic, G. Antioxidant supplements to prevent mortality. JAMA , — Antioxidant supplements and mortality. Care 17, 40— Boden, G. Excessive caloric intake acutely causes oxidative stress, GLUT4 carbonylation, and insulin resistance in healthy men. Boden, M. Overexpression of manganese superoxide dismutase ameliorates high-fat diet-induced insulin resistance in rat skeletal muscle. Bogacka, I. Structural and functional consequences of mitochondrial biogenesis in human adipocytes in vitro. Bournat, J. Mitochondrial dysfunction in obesity. Diabetes Obes. Boyle, P. Effects of pioglitazone and rosiglitazone on blood lipid levels and glycemic control in patients with type 2 diabetes mellitus: a retrospective review of randomly selected medical records. Chappuis, B. Differential effect of pioglitazone PGZ and rosiglitazone RGZ on postprandial glucose and lipid metabolism in patients with type 2 diabetes mellitus: a prospective, randomized crossover study. Diabetes Metab. Chouchani, E. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature , — Mitochondrial reactive oxygen species and adipose tissue thermogenesis: bridging physiology and mechanisms. Cornelius, N. Secondary coenzyme Q10 deficiency and oxidative stress in cultured fibroblasts from patients with riboflavin responsive multiple Acyl-CoA dehydrogenation deficiency. Cellular consequences of oxidative stress in riboflavin responsive multiple acyl-CoA dehydrogenation deficiency patient fibroblasts. Curtis, J. Protein carbonylation and metabolic control systems. Trends Endocrinol. Dasuri, K. Role of physiological levels of 4-hydroxynonenal on adipocyte biology: implications for obesity and metabolic syndrome. Free Radic. Davies, M. Protein oxidation and peroxidation. De la Mata, M. Recovery of MERRF fibroblasts and cybrids pathophysiology by coenzyme Q Neurotherapeutics 9, — Deeg, M. Pioglitazone and rosiglitazone have different effects on serum lipoprotein particle concentrations and sizes in patients with type 2 diabetes and dyslipidemia. Diabetes Care 30, — Demozay, D. FALDH reverses the deleterious action of oxidative stress induced by lipid peroxidation product 4-hydroxynonenal on insulin signaling in 3T3-L1 adipocytes. Elrayess, M. Escribano-Lopez, I. The mitochondrial antioxidant SS increases SIRT1 levels and ameliorates inflammation, oxidative stress and leukocyte-endothelium interactions in type 2 diabetes. Esterbauer, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Fazakerley, D. Mitochondrial oxidative stress causes insulin resistance without disrupting oxidative phosphorylation. Feillet-Coudray, C. The mitochondrial-targeted antioxidant MitoQ ameliorates metabolic syndrome features in obesogenic diet-fed rats better than Apocynin or Allopurinol. Fouret, G. The mitochondrial-targeted antioxidant, MitoQ, increases liver mitochondrial cardiolipin content in obesogenic diet-fed rats. Frohnert, B. Protein carbonylation, mitochondrial dysfunction, and insulin resistance. Increased adipose protein carbonylation in human obesity. Obesity 19, — Furukawa, S. Increased oxidative stress in obesity and its impact on metabolic syndrome. PubMed Abstract Google Scholar. Fusco, D. Effects of antioxidant supplementation on the aging process. Aging 2, — Gardner, P. Superoxide radical and iron modulate aconitase activity in mammalian cells. Goldberg, R. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care 28, — Goldgof, M. The chemical uncoupler 2,4-dinitrophenol DNP protects against diet-induced obesity and improves energy homeostasis in mice at thermoneutrality. Grimsrud, P. Carbonylation of adipose proteins in obesity and insulin resistance: identification of adipocyte fatty acid-binding protein as a cellular target of 4-hydroxynonenal. Proteomics 6, — Oxidative stress and covalent modification of protein with bioactive aldehydes. Han, Y. Adipocyte-specific deletion of manganese superoxide dismutase protects from diet-induced obesity through increased mitochondrial uncoupling and biogenesis. Hancock, C. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Hauck, A. Adipose oxidative stress and protein carbonylation. Obesity-induced protein carbonylation in murine adipose tissue regulates the DNA-binding domain of nuclear zinc finger proteins. Hausladen, A. Superoxide and peroxynitrite inactivate aconitases, but nitric oxide does not. Holley, A. Manganese superoxide dismutase: guardian of the powerhouse. Holloszy, J. Huh, J. Peroxiredoxin 3 is a key molecule regulating adipocyte oxidative stress, mitochondrial biogenesis, and adipokine expression. Hurd, T. Inactivation of pyruvate dehydrogenase kinase 2 by mitochondrial reactive oxygen species. Kelly, I. Effects of a thiazolidinedione compound on body fat and fat distribution of patients with type 2 diabetes. Diabetes Care 22, — Khan, M. A prospective, randomized comparison of the metabolic effects of pioglitazone or rosiglitazone in patients with type 2 diabetes who were previously treated with troglitazone. Diabetes Care 25, — Kim, J. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. King, A. A comparison in a clinical setting of the efficacy and side effects of three thiazolidinediones. Diabetes Care 23, Kowaltowski, A. Mitochondrial damage induced by conditions of oxidative stress. Lee, H. Reactive oxygen species facilitate adipocyte differentiation by accelerating mitotic clonal expansion. Mitochondrial-targeted catalase protects against high-fat diet-induced muscle insulin resistance by decreasing intramuscular lipid accumulation. Liesa, M. Mitochondrial dynamics in the regulation of nutrient utilization and energy expenditure. Cell Metab. Long, E. High-fat diet induces changes in adipose tissue transoxononenal and transhydroxynonenal levels in a depot-specific manner. Lu, R. Mitochondrial development and the influence of its dysfunction during rat adipocyte differentiation. Lushchak, O. Aconitase post-translational modification as a key in linkage between Krebs cycle, iron homeostasis, redox signaling, and metabolism of reactive oxygen species. |
| REVIEW article | Mori J, Basu R, Mclean BA, Das SK, Zhang L, Patel VB, et al. Agonist-induced hypertrophy and diastolic dysfunction are associated with selective reduction in glucose oxidation: a metabolic contribution to heart failure with normal ejection fraction. Mori J, Alrob OA, Wagg CS, Harris RA, Lopaschuk GD, Oudit GY. ANG II causes insulin resistance and induces cardiac metabolic switch and inefficiency: a critical role of PDK4. Sung MM, Das SK, Levasseur J, Byrne NJ, Fung D, Kim TT, et al. Resveratrol treatment of mice with pressure-overload-induced heart failure improves diastolic function and cardiac energy metabolism. Byrne NJ, Levasseur J, Sung MM, Masson G, Boisvenue J, Young ME, et al. Normalization of cardiac substrate utilization and left ventricular hypertrophy precede functional recovery in heart failure regression. Sung MM, Byrne NJ, Robertson IM, Kim TT, Samokhvalov V, Levasseur J, et al. Resveratrol improves exercise performance and skeletal muscle oxidative capacity in heart failure. Paolisso G, Gambardella A, Galzerano D, D'amore A, Rubino P, Verza M, et al. Total-body and myocardial substrate oxidation in congestive heart failure. Metabolism 43 —9. Swan JW, Anker SD, Walton C, Godsland IF, Clark AL, Leyva F, et al. Insulin resistance in chronic heart failure: relation to severity and etiology of heart failure. Funada J, Betts TR, Hodson L, Humphreys SM, Timperley J, Frayn KN, et al. Substrate utilization by the failing human heart by direct quantification using arterio-venous blood sampling. PLoS ONE 4 :e Slot JW, Geuze HJ, Gigengack S, James DE, Lienhard GE. Translocation of the glucose transporter GLUT4 in cardiac myocytes of the rat. Zorzano A, Sevilla L, Camps M, Becker C, Meyer J, Kammermeier H, et al. Regulation of glucose transport, and glucose transporters expression and trafficking in the heart: studies in cardiac myocytes. Lefebvre V, Méchin M-C, Louckx MP, Rider MH, Hue L. Signaling pathway involved in the activation of heart 6-phosphofructokinase by insulin. Hue L, Beauloye C, Marsin A-S, Bertrand L, Horman S, Rider MH. Insulin and ischemia stimulate glycolysis by acting on the same targets through different and opposing signaling pathways. Rider MH, Bertrand L, Vertommen D, Michels PA, Rousseau GG, Hue L. Biochem J. Lopaschuk GD, Wambolt RB, Barr RL. An imbalance between glycolysis and glucose oxidation is a possible explanation for the detrimental effects of high levels of fatty acids during aerobic reperfusion of ischemic hearts. J Pharmacol Exp Ther. Moravec J, El Alaoui-Talibi Z, Moravec M, Guendouz A. Control of oxidative metabolism in volume-overloaded rat hearts: effect of pretreatment with propionyl-L-carnitine. Adv Exp Med Biol. Kato T, Niizuma S, Inuzuka Y, Kawashima T, Okuda J, Tamaki Y, et al. Analysis of metabolic remodeling in compensated left ventricular hypertrophy and heart failure. Doenst T, Pytel G, Schrepper A, Amorim P, Färber G, Shingu Y, et al. Decreased rates of substrate oxidation ex vivo predict the onset of heart failure and contractile dysfunction in rats with pressure overload. Osorio JC, Stanley WC, Linke A, Castellari M, Diep QN, Panchal AR, et al. Impaired myocardial fatty acid oxidation and reduced protein expression of retinoid X receptor-alpha in pacing-induced heart failure. Kolwicz SC Jr, Olson DP, Marney LC, Garcia-Menendez L, Synovec RE, Tian R. Cardiac-specific deletion of acetyl CoA carboxylase 2 prevents metabolic remodeling during pressure-overload hypertrophy. Masoud WGT, Ussher JR, Wang W, Jaswal JS, Wagg CS, Dyck JR, et al. Failing mouse hearts utilize energy inefficiently and benefit from improved coupling of glycolysis and glucose oxidation. Recchia FA, Mcconnell PI, Bernstein RD, Vogel TR, Xu X, Hintze TH. Reduced nitric oxide production and altered myocardial metabolism during the decompensation of pacing-induced heart failure in the conscious dog. Bround MJ, Wambolt R, Cen H, Asghari P, Albu RF, Han J, et al. Cardiac ryanodine receptor Ryr2 -mediated calcium signals specifically promote glucose oxidation via pyruvate dehydrogenase. Gupte AA, Hamilton DJ, Cordero-Reyes AM, Youker KA, Yin Z, Estep JD, et al. Mechanical unloading promotes myocardial energy recovery in human heart failure. Circ Cardiovasc Genet. Ussher JR, Koves TR, Jaswal JS, Zhang L, Ilkayeva O, Dyck JRB, et al. Insulin-stimulated cardiac glucose oxidation is increased in high-fat diet—induced obese mice lacking malonyl CoA decarboxylase. Diabetes 58 — Sankaralingam S, Abo Alrob O, Zhang L, Jaswal JS, Wagg CS, Fukushima A, et al. Lowering body weight in obese mice with diastolic heart failure improves cardiac insulin sensitivity and function: implications for the obesity paradox. Diabetes 64 — Ho KL, Ussher JR. Cardiac energy metabolism in diabetes. Heart Metab. Abel ED, Kaulbach HC, Tian R, Hopkins JC, Duffy J, Doetschman T, et al. Cardiac hypertrophy with preserved contractile function after selective deletion of GLUT4 from the heart. Rutter MK, Parise H, Benjamin EJ, Levy D, Larson MG, Meigs JB, et al. Impact of glucose intolerance and insulin resistance on cardiac structure and function: sex-related differences in the Framingham Heart Study. Peterson LR, Herrero P, Schechtman KB, Racette SB, Waggoner AD, Kisrieva-Ware Z, et al. Effect of obesity and insulin resistance on myocardial substrate metabolism and efficiency in young women. Le Page LM, Rider OJ, Lewis AJ, Ball V, Clarke K, Johansson E, et al. Increasing pyruvate dehydrogenase flux as a treatment for diabetic cardiomyopathy: a combined 13C hyperpolarized magnetic resonance and echocardiography study. Gopal K, Almutairi M, Al Batran R, Eaton F, Gandhi M, Ussher JR. Cardiac-specific deletion of pyruvate dehydrogenase impairs glucose oxidation rates and induces diastolic dysfunction. Front Cardiovasc Med. Horton JL, Martin OJ, Lai L, Riley NM, Richards AL, Vega RB, et al. Mitochondrial protein hyperacetylation in the failing heart. JCI Insight 1 :e Ozden O, Park SH, Wagner BA, Song HY, Zhu Y, Vassilopoulos A, et al. SIRT3 deacetylates and increases pyruvate dehydrogenase activity in cancer cells. Free Radic Biol Med. Zhang X, Ji R, Liao X, Deng LY, Castillero E, Givens R, et al. Abstract lysine acetylation of pyruvate dehydrogenase reduces enzymatic activity and contributes to impaired substrate metabolism in the failing myocardium. Circulation :A Alrob OA, Sankaralingam S, Ma C, Wagg CS, Fillmore N, Jaswal JS, et al. Obesity-induced lysine acetylation increases cardiac fatty acid oxidation and impairs insulin signalling. Khan D, Sarikhani M, Dasgupta S, Maniyadath B, Pandit AS, Mishra S, et al. SIRT6 deacetylase transcriptionally regulates glucose metabolism in heart. J Cell Physiol. Cardiac metabolism as a target for the treatment of heart failure. Huss JM, Kelly DP. Mitochondrial energy metabolism in heart failure: a question of balance. Ingwall JS. Energy metabolism in heart failure and remodelling. Sack MN, Rader TA, Park S, Bastin J, Mccune SA, Kelly DP. Fatty acid oxidation enzyme gene expression is downregulated in the failing heart. Circulation 94 — Sack MN, Disch DL, Rockman HA, Kelly DP. A role for Sp and nuclear receptor transcription factors in a cardiac hypertrophic growth program. Proc Natl Acad Sci. Rajabi M, Kassiotis C, Razeghi P, Taegtmeyer H. Return to the fetal gene program protects the stressed heart: a strong hypothesis. Lommi J, Kupari M, Yki-Järvinen H. Free fatty acid kinetics and oxidation in congestive heart failure. Nørrelund H, Wiggers H, Halbirk M, Frystyk J, Flyvbjerg A, Bøtker HE, et al. Abnormalities of whole body protein turnover, muscle metabolism and levels of metabolic hormones in patients with chronic heart failure. J Int Med. Tuunanen H, Engblom E, Naum A, Scheinin M, Någren K, Airaksinen J, et al. Decreased myocardial free fatty acid uptake in patients with idiopathic dilated cardiomyopathy: evidence of relationship with insulin resistance and left ventricular dysfunction. J Card Fail. Tuunanen H, Ukkonen H, Knuuti J. Myocardial fatty acid metabolism and cardiac performance in heart failure. Curr Cardiol Rep. Taylor M, Wallhaus TR, Degrado TR, Russell DC, Stanko P, Nickles RJ, et al. An evaluation of myocardial fatty acid and glucose uptake using PET with [18F] fluorothia-heptadecanoic acid and [ 18 F] FDG in patients with congestive heart failure. J Nucl Med. Lei B, Lionetti V, Young ME, Chandler MP, D'agostino C, Kang E, et al. Paradoxical downregulation of the glucose oxidation pathway despite enhanced flux in severe heart failure. Qanud K, Mamdani M, Pepe M, Khairallah RJ, Gravel J, Lei B, et al. Reverse changes in cardiac substrate oxidation in dogs recovering from heart failure. Lopaschuk GD, Tsang H. Mazumder PK, O'neill BT, Roberts MW, Buchanan J, Yun UJ, Cooksey RC, et al. Diabetes 53 — Buchanan J, Mazumder PK, Hu P, Chakrabarti G, Roberts MW, Yun UJ, et al. Reduced cardiac efficiency and altered substrate metabolism precedes the onset of hyperglycemia and contractile dysfunction in two mouse models of insulin resistance and obesity. Endocrinology —9. Lin CH, Kurup S, Herrero P, Schechtman KB, Eagon JC, Klein S, et al. Myocardial oxygen consumption change predicts left ventricular relaxation improvement in obese humans after weight loss. Obesity 19 — Tuunanen H, Engblom E, Naum A, Nagren K, Hesse B, Airaksinen KE, et al. Free fatty acid depletion acutely decreases cardiac work and efficiency in cardiomyopathic heart failure. Circulation —7. White M, Kahn C. The insulin signaling system. J biol Chem. Fukushima A, Lopaschuk GD. Cardiac fatty acid oxidation in heart failure associated with obesity and diabetes. Biochim Biophys Acta Mol Cell Biol Lipids — Itani SI, Ruderman NB, Schmieder F, Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IκB-α. Diabetes 51 — Finck BN, Kelly DP. Peroxisome proliferator—activated receptor γ coactivator-1 PGC-1 regulatory cascade in cardiac physiology and disease. Madrazo JA, Kelly DP. The PPAR trio: regulators of myocardial energy metabolism in health and disease. Kanda H, Nohara R, Hasegawa K, Kishimoto C, Sasayama S. Heart Vessels 15 —6. Nuclear receptor signaling and cardiac energetics. PGC-1 coactivators: inducible regulators of energy metabolism in health and disease. Hopkins TA, Sugden MC, Holness MJ, Kozak R, Dyck JR, Lopaschuk GD. Control of cardiac pyruvate dehydrogenase activity in peroxisome proliferator-activated receptor-α transgenic mice. Schummer CM, Werner U, Tennagels N, Schmoll D, Haschke G, Juretschke HP, et al. Dysregulated pyruvate dehydrogenase complex in Zucker diabetic fatty rats. Karbowska J, Kochan Z, Smolenski RT. Peroxisome proliferator-activated receptor alpha is downregulated in the failing human heart. Cell Mol Biol Lett. Arany Z, Novikov M, Chin S, Ma Y, Rosenzweig A, Spiegelman BM. Transverse aortic constriction leads to accelerated heart failure in mice lacking PPAR-γ coactivator 1α. Karamanlidis G, Nascimben L, Couper GS, Shekar PS, Del Monte F, Tian R. Defective DNA replication impairs mitochondrial biogenesis in human failing hearts. Sihag S, Cresci S, Li AY, Sucharov CC, Lehman JJ. PGC-1α and ERRα target gene downregulation is a signature of the failing human heart. Xiong Y, Guan K-L. Mechanistic insights into the regulation of metabolic enzymes by acetylation. J Cell Biol. Webster BR, Scott I, Han K, Li JH, Lu Z, Stevens MV, et al. Restricted mitochondrial protein acetylation initiates mitochondrial autophagy. J Cell Sci. Schwer B, Verdin E. Conserved metabolic regulatory functions of sirtuins. Newman JC, He W, Verdin E. Mitochondrial protein acylation and intermediary metabolism: regulation by sirtuins and implications for metabolic disease. Zhao S, Xu W, Jiang W, Yu W, Lin Y, Zhang T, et al. Regulation of cellular metabolism by protein lysine acetylation. Science —4. Murray AJ, Anderson RE, Watson GC, Radda GK, Clarke K. Uncoupling proteins in human heart. Lancet —8. Kim G, Jo K, Kim KJ, Lee Y-H, Han E, Yoon H-J, et al. Visceral adiposity is associated with altered myocardial glucose uptake measured by 18FDG-PET in subjects with normal glucose tolerance, prediabetes, and type 2 diabetes. Cardiovasc Diabetol. Li C, Lu C, Zhao X, Chen X. Comparison between myocardial infarction and diabetes mellitus damage caused angiogenesis or energy metabolism. Int J Clin Exp Med. Murray AJ, Cole MA, Lygate CA, Carr CA, Stuckey DJ, Little SE, et al. Increased mitochondrial uncoupling proteins, respiratory uncoupling and decreased efficiency in the chronically infarcted rat heart. Lommi J, Kupari M, Koskinen P, Näveri H, Leinonen H, Pulkki K, et al. Blood ketone bodies in congestive heart failure. Lommi J, Koskinen P, Näveri H, Härkönen M, Kupari M. Heart failure ketosis. Melenovsky V, Kotrc M, Polak J, Pelikanova T, Bendlova B, Cahova M, et al. Availability of energetic substrates and exercise performance in heart failure with or without diabetes. Du Z, Shen A, Huang Y, Su L, Lai W, Wang P, et al. Zordoky BN, Sung MM, Ezekowitz J, Mandal R, Han B, Bjorndahl TC, et al. Metabolomic fingerprint of heart failure with preserved ejection fraction. PLoS ONE 10 :e Kolwicz SC, Airhart S, Tian R. Ketones step to the plate: a game changer for metabolic remodeling in heart failure? Ikegami R, Shimizu I, Yoshida Y, Minamino T. Metabolomic analysis in heart failure. Circ J. Robinson AM, Williamson DH. Physiological roles of ketone bodies as substrates and signals in mammalian tissues. Janardhan A, Chen J, Crawford PA. Altered systemic ketone body metabolism in advanced heart failure. Tex Heart Inst J. Nagao M, Toh R, Irino Y, Mori T, Nakajima H, Hara T, et al. β-Hydroxybutyrate elevation as a compensatory response against oxidative stress in cardiomyocytes. Biochem Biophys Res Commun. Shimazu T, Hirschey MD, Newman J, He W, Shirakawa K, Le Moan N, et al. Suppression of oxidative stress by β-hydroxybutyrate, an endogenous histone deacetylase inhibitor. Schugar RC, Moll AR, André D'avignon D, Weinheimer CJ, Kovacs A, Crawford PA. Cardiomyocyte-specific deficiency of ketone body metabolism promotes accelerated pathological remodeling. Mol Metab. Uchihashi M, Hoshino A, Okawa Y, Ariyoshi M, Kaimoto S, Tateishi S, et al. Cardiac-specific Bdh1 overexpression ameliorates oxidative stress and cardiac remodeling in pressure overload-induced heart failure. Seki M, Powers JC, Maruyama S, Zuriaga MA, Wu CL, Kurishima C, et al. Acute and chronic increases of circulating FSTL1 normalize energy substrate metabolism in pacing-induced heart failure. Jeong MY, Lin YH, Wennersten SA, Demos-Davies KM, Cavasin MA, Mahaffey JH, et al. Histone deacetylase activity governs diastolic dysfunction through a nongenomic mechanism. Sci Transl Med. Al Batran R, Ussher JR. Revisiting protein acetylation and myocardial fatty acid oxidation. Williamson JR, Krebs HA. Acetoacetate as fuel of respiration in the perfused rat heart. Wieland O, Funcke HV, Löffler G. Interconversion of pyruvate dehydrogenase in rat heart muscle upon perfusion with fatty acids or ketone bodies. FEBS Lett. Hiltunen JK, Hassinen IE. Energy-linked regulation of glucose and pyruvate oxidation in isolated perfused rat heart. Role of pyruvate dehydrogenase. Biochim Biophys Acta Bioenerget. Kerbey AL, Randle PJ, Cooper RH, Whitehouse S, Pask HT, Denton RM. Regulation of pyruvate dehydrogenase in rat heart. Mechanism of regulation of proportions of dephosphorylated and phosphorylated enzyme by oxidation of fatty acids and ketone bodies and of effects of diabetes: role of coenzyme A, acetyl-coenzyme A and reduced and oxidized nicotinamide-adenine dinucleotide. Gormsen LC, Svart M, Thomsen HH, Sondergaard E, Vendelbo MH, Christensen N, et al. Ketone body infusion with 3-hydroxybutyrate reduces myocardial glucose uptake and increases blood flow in humans: a positron emission tomography study. J Am Heart Assoc. Renguet E, Ginion A, Gelinas R, Bultot L, Auquier J, Robillard Frayne I, et al. Metabolism and acetylation contribute to leucine-mediated inhibition of cardiac glucose uptake. Kashiwaya Y, King MT, Veech RL. Substrate signaling by insulin: a ketone bodies ratio mimics insulin action in heart. Chen V, Wagner G, Spitzer JJ. Regulation of substrate oxidation in isolated myocardial cells by beta-hydroxybutyrate. Horm Metab Res. Forsey RG, Reid K, Brosnan JT. Competition between fatty acids and carbohydrate or ketone bodies as metabolic fuels for the isolated perfused heart. Can J Physiol Pharmacol. Stanley WC, Meadows SR, Kivilo KM, Roth BA, Lopaschuk GD. beta-Hydroxybutyrate inhibits myocardial fatty acid oxidation in vivo independent of changes in malonyl-CoA content. Liao R, Jain M, Cui L, D'agostino J, Aiello F, Luptak I, et al. Cardiac-specific overexpression of GLUT1 prevents the development of heart failure attributable to pressure overload in mice. Liu B, Clanachan AS, Schulz R, Lopaschuk GD. Cardiac efficiency is improved after ischemia by altering both the source and fate of protons. Lydell CP, Chan A, Wambolt RB, Sambandam N, Parsons H, Bondy GP, et al. Pyruvate dehydrogenase and the regulation of glucose oxidation in hypertrophied rat hearts. Wargovich TJ, Macdonald RG, Hill JA, Feldman RL, Stacpoole PW, Pepine CJ. Myocardial metabolic and hemodynamic effects of dichloroacetate in coronary artery disease. Bersin RM, Wolfe C, Kwasman M, Lau D, Klinski C, Tanaka K, et al. Improved hemodynamic function and mechanical efficiency in congestive heart failure with sodium dichloroacetate. Lewis JF, Dacosta M, Wargowich T, Stacpoole P. Effects of dichloroacetate in patients with congestive heart failure. Clin Cardiol. Lopaschuk GD, Wall SR, Olley PM, Davies NJ. Etomoxir, a carnitine palmitoyltransferase I inhibitor, protects hearts from fatty acid-induced ischemic injury independent of changes in long chain acylcarnitine. Hajri T, Han XX, Bonen A, Abumrad NA. Defective fatty acid uptake modulates insulin responsiveness and metabolic responses to diet in CDnull mice. Schmidt-Schweda S, Holubarsch C. First clinical trial with etomoxir in patients with chronic congestive heart failure. Clin Sci. Holubarsch CJ, Rohrbach M, Karrasch M, Boehm E, Polonski L, Ponikowski P, et al. A double-blind randomized multicentre clinical trial to evaluate the efficacy and safety of two doses of etomoxir in comparison with placebo in patients with moderate congestive heart failure: the ERGO etomoxir for the recovery of glucose oxidation study. Lee L, Campbell R, Scheuermann-Freestone M, Taylor R, Gunaruwan P, Williams L, et al. Metabolic modulation with perhexiline in chronic heart failure: a randomized, controlled trial of short-term use of a novel treatment. Coort SL, Willems J, Coumans WA, Van Der Vusse GJ, Bonen A, Glatz JF, et al. Kantor PF, Lucien A, Kozak R, Lopaschuk GD. The antianginal drug trimetazidine shifts cardiac energy metabolism from fatty acid oxidation to glucose oxidation by inhibiting mitochondrial long-chain 3-ketoacyl coenzyme A thiolase. Peng S, Zhao M, Wan J, Fang Q, Fang D, Li K. The efficacy of trimetazidine on stable angina pectoris: a meta-analysis of randomized clinical trials. Int J Cardiol. Fragasso G, Palloshi A, Puccetti P, Silipigni C, Rossodivita A, Pala M, et al. A randomized clinical trial of trimetazidine, a partial free fatty acid oxidation inhibitor, in patients with heart failure. Gao D, Ning N, Niu X, Hao G, Meng Z. Trimetazidine: a meta-analysis of randomised controlled trials in heart failure. Heart 97 — Ussher JR, Fillmore N, Keung W, Mori J, Beker DL, Wagg CS, et al. Trimetazidine therapy prevents obesity-induced cardiomyopathy in mice. Canad J Cardiol. Ussher JR, Keung W, Fillmore N, Koves TR, Mori J, Zhang L, et al. Treatment with the 3-ketoacyl-CoA thiolase inhibitor trimetazidine does not exacerbate whole-body insulin resistance in obese mice. Chaitman BR, Pepine CJ, Parker JO, Skopal J, Chumakova G, Kuch J, et al. Effects of ranolazine with atenolol, amlodipine, or diltiazem on exercise tolerance and angina frequency in patients with severe chronic angina: a randomized controlled trial. Scirica BM, Morrow DA, Hod H, Murphy SA, Belardinelli L, Hedgepeth CM, et al. Effect of ranolazine, an antianginal agent with novel electrophysiological properties, on the incidence of arrhythmias in patients with non—ST-segment—elevation acute coronary syndrome: results from the Metabolic Efficiency with Ranolazine for Less Ischemia in Non—ST-Elevation Acute Coronary Syndrome—Thrombolysis in Myocardial Infarction 36 MERLIN-TIMI 36 randomized controlled trial. Mccormack JG, Barr RL, Wolff AA, Lopaschuk GD. Ranolazine stimulates glucose oxidation in normoxic, ischemic, and reperfused ischemic rat hearts. Circulation 93 — Ferrannini E, Mark M, Mayoux E. Diabetes Care 39 — Mudaliar S, Alloju S, Henry RR. Can a shift in fuel energetics explain the beneficial cardiorenal outcomes in the EMPA-REG OUTCOME study? A unifying hypothesis. Zinman B, Wanner C, Lachin JM, Fitchett D, Bluhmki E, Hantel S, et al. Empagliflozin, Cardiovascular outcomes, and mortality in type 2 diabetes. Byrne NJ, Parajuli N, Levasseur JL, Boisvenue J, Beker D, Masson G, et al. Empagliflozin prevents worsening of cardiac function in an experimental model of pressure overload-induced heart failure. Lopaschuk GD, Verma S. Empagliflozin's fuel hypothesis: not so soon. Cell Metabolism 24 —2. Stanley WC, Morgan EE, Huang H, Mcelfresh TA, Sterk JP, Okere IC, et al. Malonyl-CoA decarboxylase inhibition suppresses fatty acid oxidation and reduces lactate production during demand-induced ischemia. Dyck JRB, Cheng J-F, Stanley WC, Barr R, Chandler MP, Brown S, et al. Malonyl coenzyme a decarboxylase inhibition protects the ischemic heart by inhibiting fatty acid oxidation and stimulating glucose oxidation. Dai W, Shi J, Gupta RC, Sabbah HN, Hale SL, Kloner RA. Bendavia, a mitochondria-targeting peptide, improves postinfarction cardiac function, prevents adverse left ventricular remodeling, and restores mitochondria-related gene expression in rats. J Cardiovasc Pharmacol. Karwi QG, Bornbaum J, Boengler K, Torregrossa R, Whiteman M, Wood ME, et al. AP39, a mitochondria-targeting hydrogen sulfide H2S donor, protects against myocardial reperfusion injury independently of salvage kinase signalling. Br J Pharmacol. Sabbah HN, Gupta RC, Kohli S, Wang M, Hachem S, Zhang K. Chronic Therapy With Elamipretide MTP , a novel mitochondria-targeting peptide, improves left ventricular and mitochondrial function in dogs with advanced heart failure. Daubert MA, Yow E, Dunn G, Marchev S, Barnhart H, Douglas PS, et al. Novel mitochondria-targeting peptide in heart failure treatment. A randomized, placebo-controlled trial of elamipretide Circ Heart Fail. Edes I, Kiss E, Kitada Y, Powers FM, Papp JG, Kranias EG, et al. Save this study. Warning You have reached the maximum number of saved studies Brown Fat Activation and Browning Efficiency Augmented by Chronic Cold and Nutraceuticals for Brown Adipose Tissue-mediated Effect Against Metabolic Syndrome BEACON BEAMS Study The safety and scientific validity of this study is the responsibility of the study sponsor and investigators. Listing a study does not mean it has been evaluated by the U. Federal Government. Know the risks and potential benefits of clinical studies and talk to your health care provider before participating. Read our disclaimer for details. gov Identifier: NCT Recruitment Status : Recruiting First Posted : October 20, Last Update Posted : February 23, See Contacts and Locations. View this study on the modernized ClinicalTrials. Singapore Institute of Food and Biotechnology Innovation SIFBI. Melvin Leow, Singapore Institute for Clinical Sciences. Study Details Tabular View No Results Posted Disclaimer How to Read a Study Record. Study Description. Go to Top of Page Study Description Study Design Arms and Interventions Outcome Measures Eligibility Criteria Contacts and Locations More Information. Show detailed description. Hide detailed description. Detailed Description:. The participants will then be required to come for the 4th visit third test session and 5th visit fourth test session at week 12 after completing the 3 months of intervention , as per follows: On the 4th visit third test session at week 12, participants will repeat the same procedures as per that of the 2nd visit first test session. Resource links provided by the National Library of Medicine MedlinePlus related topics: Metabolic Syndrome. Genetic and Rare Diseases Information Center resources: Chronic Graft Versus Host Disease. FDA Resources. Arms and Interventions. Subjects will consume mg of curcumin daily for the next 12 weeks or 3 months. Subject will consume mg of curcumin a naturally-occurring polyphenol antioxidant that is found in turmeric ginger rhizome root. Subjects will undergo a mild cold stimulation of about 14 degrees Celsius by wearing a cooling vest for approximately an hour and consume mg of curcumin daily for the next 12 weeks or 3 months. Subject wear a cooling vest and consume mg of curcumin. Outcome Measures. Eligibility Criteria. Information from the National Library of Medicine Choosing to participate in a study is an important personal decision. Layout table for eligibility information Ages Eligible for Study: 21 Years to 50 Years Adult Sexes Eligible for Study: All Accepts Healthy Volunteers: Yes Criteria. anaphylaxis to peanuts Having active Tuberculosis TB or currently receiving treatment for TB Have any known Chronic Infection or known to suffer from or have previously suffered from or is a carrier of Hepatitis B Virus HBV , Hepatitis C Virus HCV , Human Immunodeficiency Virus HIV Are a member of the research team or their immediate family members. Immediate family member is defined as a spouse, parent, child, or sibling, whether biological or legally adopted. Enrolled in a concurrent research study judged not to be scientifically or medically compatible with the study of the CNRC Have poor veins impeding venous access Have any history of severe vasovagal syncope blackouts or near faints following blood draws History of surgery with metallic clips, staples or stents Presence of cardiac pacemaker or other foreign body in any part of the body History of claustrophobia particularly in a MRI scanner. Contacts and Locations. Information from the National Library of Medicine To learn more about this study, you or your doctor may contact the study research staff using the contact information provided by the sponsor. Please refer to this study by its ClinicalTrials. gov identifier NCT number : NCT More Information. Layout table for additonal information Responsible Party: Melvin Leow, Principal Investigator, Singapore Institute for Clinical Sciences ClinicalTrials. FDA-regulated Drug Product: No Studies a U. FDA-regulated Device Product: No Additional relevant MeSH terms:. Layout table for MeSH terms Metabolic Syndrome Insulin Resistance Hyperinsulinism Glucose Metabolism Disorders Metabolic Diseases. For Patients and Families For Researchers For Study Record Managers. The increasing prevalence of obesity suggests lifestyle intervention as the principal method to treat obesity is unlikely to succeed. Currently, all available anti-obesity medications act by limiting energy intake through appetite suppression or inhibition of intestinal lipid absorption. However, these medications are largely ineffective and often have adverse side effects. The central role of mitochondria in nutrient handling provides a logical entry point for improving metabolism in obesity. While approaches to understanding and intervening in oxidative damage evolve, exploration of mitochondria redox balance may enable development of dietary and small molecule therapies for obesity and its comorbidities. This work was funded by the American Diabetes Association IBS and NIH R01DK The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest. Acosta, M. Coenzyme Q biosynthesis in health and disease. Acta , — doi: PubMed Abstract CrossRef Full Text Google Scholar. Ahmed, M. Proteomic analysis of human adipose tissue after rosiglitazone treatment shows coordinated changes to promote glucose uptake. Obesity 18, 27— Akl, M. Perturbed adipose tissue hydrogen peroxide metabolism in centrally obese men: association with insulin resistance. PLoS One e Anderson, E. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. Armstrong, J. The redox regulation of intermediary metabolism by a superoxide-aconitase rheostat. Bioessays 26, — Barbosa, M. Hydrogen peroxide production regulates the mitochondrial function in insulin resistant muscle cells: effect of catalase overexpression. Bjelakovic, G. Antioxidant supplements to prevent mortality. JAMA , — Antioxidant supplements and mortality. Care 17, 40— Boden, G. Excessive caloric intake acutely causes oxidative stress, GLUT4 carbonylation, and insulin resistance in healthy men. Boden, M. Overexpression of manganese superoxide dismutase ameliorates high-fat diet-induced insulin resistance in rat skeletal muscle. Bogacka, I. Structural and functional consequences of mitochondrial biogenesis in human adipocytes in vitro. Bournat, J. Mitochondrial dysfunction in obesity. Diabetes Obes. Boyle, P. Effects of pioglitazone and rosiglitazone on blood lipid levels and glycemic control in patients with type 2 diabetes mellitus: a retrospective review of randomly selected medical records. Chappuis, B. Differential effect of pioglitazone PGZ and rosiglitazone RGZ on postprandial glucose and lipid metabolism in patients with type 2 diabetes mellitus: a prospective, randomized crossover study. Diabetes Metab. Chouchani, E. Mitochondrial ROS regulate thermogenic energy expenditure and sulfenylation of UCP1. Nature , — Mitochondrial reactive oxygen species and adipose tissue thermogenesis: bridging physiology and mechanisms. Cornelius, N. Secondary coenzyme Q10 deficiency and oxidative stress in cultured fibroblasts from patients with riboflavin responsive multiple Acyl-CoA dehydrogenation deficiency. Cellular consequences of oxidative stress in riboflavin responsive multiple acyl-CoA dehydrogenation deficiency patient fibroblasts. Curtis, J. Protein carbonylation and metabolic control systems. Trends Endocrinol. Dasuri, K. Role of physiological levels of 4-hydroxynonenal on adipocyte biology: implications for obesity and metabolic syndrome. Free Radic. Davies, M. Protein oxidation and peroxidation. De la Mata, M. Recovery of MERRF fibroblasts and cybrids pathophysiology by coenzyme Q Neurotherapeutics 9, — Deeg, M. Pioglitazone and rosiglitazone have different effects on serum lipoprotein particle concentrations and sizes in patients with type 2 diabetes and dyslipidemia. Diabetes Care 30, — Demozay, D. FALDH reverses the deleterious action of oxidative stress induced by lipid peroxidation product 4-hydroxynonenal on insulin signaling in 3T3-L1 adipocytes. Elrayess, M. Escribano-Lopez, I. The mitochondrial antioxidant SS increases SIRT1 levels and ameliorates inflammation, oxidative stress and leukocyte-endothelium interactions in type 2 diabetes. Esterbauer, H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Fazakerley, D. Mitochondrial oxidative stress causes insulin resistance without disrupting oxidative phosphorylation. Feillet-Coudray, C. The mitochondrial-targeted antioxidant MitoQ ameliorates metabolic syndrome features in obesogenic diet-fed rats better than Apocynin or Allopurinol. Fouret, G. The mitochondrial-targeted antioxidant, MitoQ, increases liver mitochondrial cardiolipin content in obesogenic diet-fed rats. Frohnert, B. Protein carbonylation, mitochondrial dysfunction, and insulin resistance. Increased adipose protein carbonylation in human obesity. Obesity 19, — Furukawa, S. Increased oxidative stress in obesity and its impact on metabolic syndrome. PubMed Abstract Google Scholar. Fusco, D. Effects of antioxidant supplementation on the aging process. Aging 2, — Gardner, P. Superoxide radical and iron modulate aconitase activity in mammalian cells. Goldberg, R. A comparison of lipid and glycemic effects of pioglitazone and rosiglitazone in patients with type 2 diabetes and dyslipidemia. Diabetes Care 28, — Goldgof, M. The chemical uncoupler 2,4-dinitrophenol DNP protects against diet-induced obesity and improves energy homeostasis in mice at thermoneutrality. Grimsrud, P. Carbonylation of adipose proteins in obesity and insulin resistance: identification of adipocyte fatty acid-binding protein as a cellular target of 4-hydroxynonenal. Proteomics 6, — Oxidative stress and covalent modification of protein with bioactive aldehydes. Han, Y. Adipocyte-specific deletion of manganese superoxide dismutase protects from diet-induced obesity through increased mitochondrial uncoupling and biogenesis. Hancock, C. High-fat diets cause insulin resistance despite an increase in muscle mitochondria. Hauck, A. Adipose oxidative stress and protein carbonylation. Obesity-induced protein carbonylation in murine adipose tissue regulates the DNA-binding domain of nuclear zinc finger proteins. Hausladen, A. Superoxide and peroxynitrite inactivate aconitases, but nitric oxide does not. Holley, A. Manganese superoxide dismutase: guardian of the powerhouse. Holloszy, J. Huh, J. Peroxiredoxin 3 is a key molecule regulating adipocyte oxidative stress, mitochondrial biogenesis, and adipokine expression. Hurd, T. Inactivation of pyruvate dehydrogenase kinase 2 by mitochondrial reactive oxygen species. Kelly, I. Effects of a thiazolidinedione compound on body fat and fat distribution of patients with type 2 diabetes. Diabetes Care 22, — Khan, M. A prospective, randomized comparison of the metabolic effects of pioglitazone or rosiglitazone in patients with type 2 diabetes who were previously treated with troglitazone. Diabetes Care 25, — Kim, J. Obesity-associated improvements in metabolic profile through expansion of adipose tissue. King, A. A comparison in a clinical setting of the efficacy and side effects of three thiazolidinediones. Diabetes Care 23, Kowaltowski, A. |
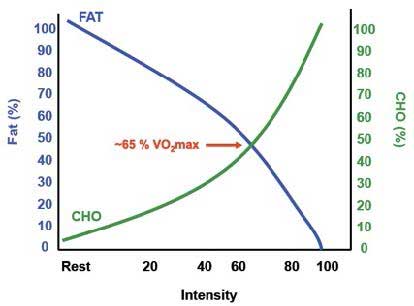
Die Kleinigkeiten!
Sie lassen den Fehler zu. Geben Sie wir werden besprechen. Schreiben Sie mir in PM.
Ich tue Abbitte, dass ich mich einmische, ich wollte die Meinung auch aussprechen.
sogar so
Wie man es bestimmen kann?